-
iestinstrument
Pressure Effect on Mechanical and Electrochemical Properties of Lithium Cobalt Oxide Powder Materials
Literature Appreciation: Pressure Effect on Mechanical and Electrochemical Properties of Lithium Cobalt Oxide Powder Materials
1. Article Overview
The design, manufacture and handling of active substance powders have a huge impact on battery performance. The consistency of the active substance powder before mass production ensures the stability of the battery performance. The performance of powders depends on composition, state of encapsulation, compressibility and flowability. On the mesoscopic scale, the cohesion between powders and the adhesion to the substrate have a greater influence on the stacking state than the particle morphology. In order to maintain good flowability, the presence of aggregates or lumps in the powder should be avoided, as powder agglomeration leads to partial arching and tumbling, which increases resistivity. After the pressurization and depressurization steps of the upper press, the loose powder is compacted into round pieces. Electrode powders undergo rearrangement, elastic deformation, plastic deformation and particle fragmentation during compaction, accompanied by changes in mechanical and electrochemical properties. The above mechanisms usually occur overlappingly, but not sequentially. When a compressive force is applied to the top of the powder, the void ratio of the powder can be reduced from about 40% to 1%, but the relationship between the compressive force and the compacted density is not clear. Therefore, it is necessary to investigate the effect of pressure on the mechanical and electrical properties of cathode materials.
2. Sample Preparation and Testing
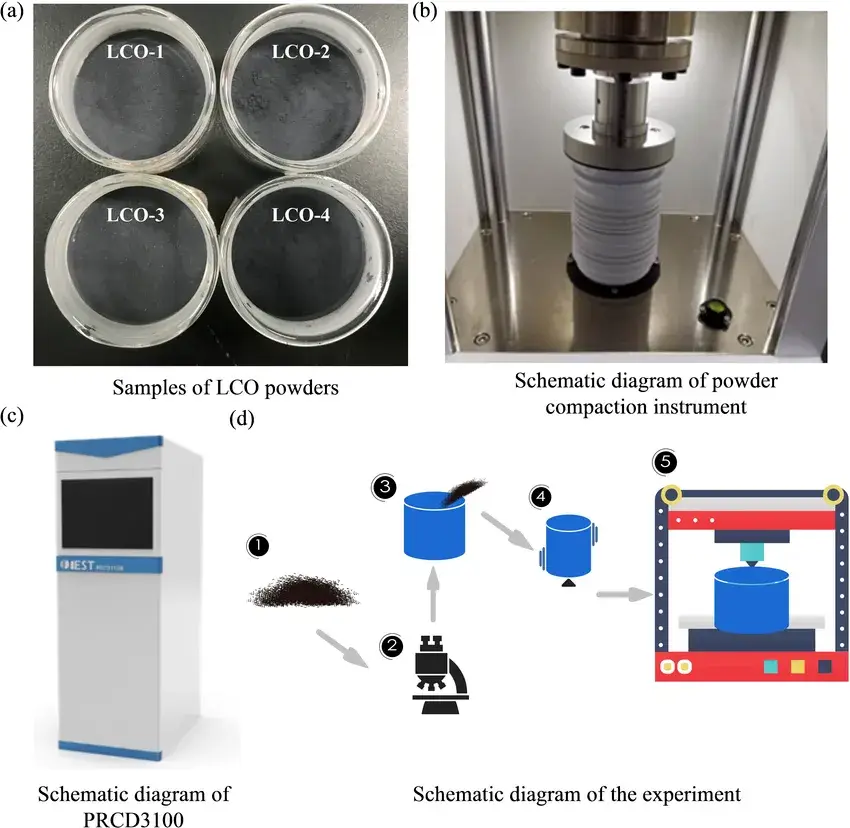
In this paper, the elastic-plastic deformation and electrical resistance properties of various lithium cobalt oxide(LCO) powders during compaction were investigated using a powder resistivity testing system. As shown in Figure. 1, the stress-strain, compaction density and electrical resistance of lithium cobalt oxide(LCO) powders during loading and unloading were measured in real time using the Powder Resistivity & Compaction Density Tester (PRCD3100) from IEST. The electrochemical properties of lithium cobalt oxide(LCO) electrodes under different compaction methods were also compared. Material characterization, compaction and electrochemical tests revealed the relationship between mechanical and electrochemical properties of electrode particles.
Figure 1. Schematic diagram of experimental materials and instruments: (a) 4 different LCO samples; (b) schematic diagram of the interior of the powder compaction detector; (c) schematic diagram of the exterior of the powder compaction detector; (d) powder compaction experimental procedure.
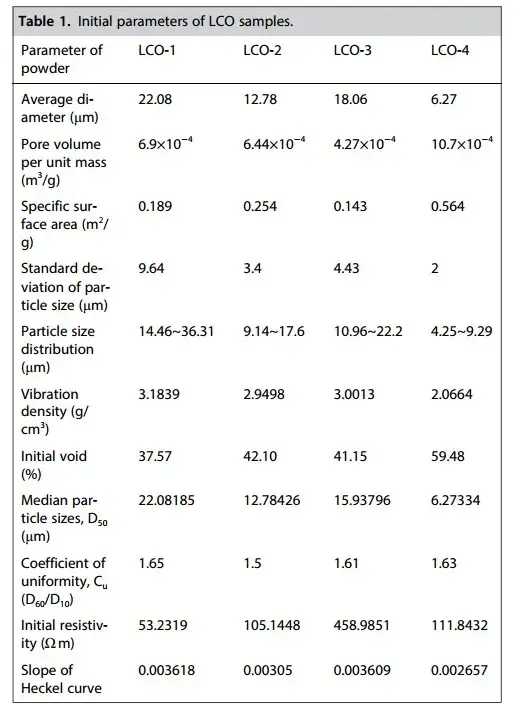
In this paper, four LCO powders were selected for investigation, and their physicochemical properties are shown in Table 1; most of the parameters are in the order of LCO-1> LCO-3> LCO-2> LCO-4, of which, the size of the particle size is in the order of LCO-1> LCO-3> LCO-2> LCO-4. And the uniformity of the powders was identified using D60/D10 to quantify the homogeneity of the particle size distribution. The specific surface area was closely related to the particle size, with the size of LCO4>LCO-2>LCO-1>LCO-3.
Table 1. Initial physicochemical properties of four lithium cobalt oxide(LCO) powders
3. Analysis of results
Figures 2a-d show the overall and localized SEM images of lithium cobalt oxide powders at 50, 100, 150, and 200 MPa, respectively. The differences in the particle size distribution of the powders directly affect the filling efficiency, compaction density and electrochemical properties of the powders during compression. Compared with other specimens, the LCO-4 specimen with the smallest average particle size has less particle deformation and is less fragile under the same pressure. It can be seen that LCO powder undergoes shear fracture under certain force. When the shear stress on any plane is equal to the shear strength of LCO, the surface cracking occurs. During shear fracture, a set of inclined tensile cracks appear, and as the stress increases, the tensile cracks penetrate into each other to form a penetrating shear surface, which ultimately leads to the final shear fracture of the particle. It is worth noting that fracture is only effective for secondary particles consisting of primary particles.
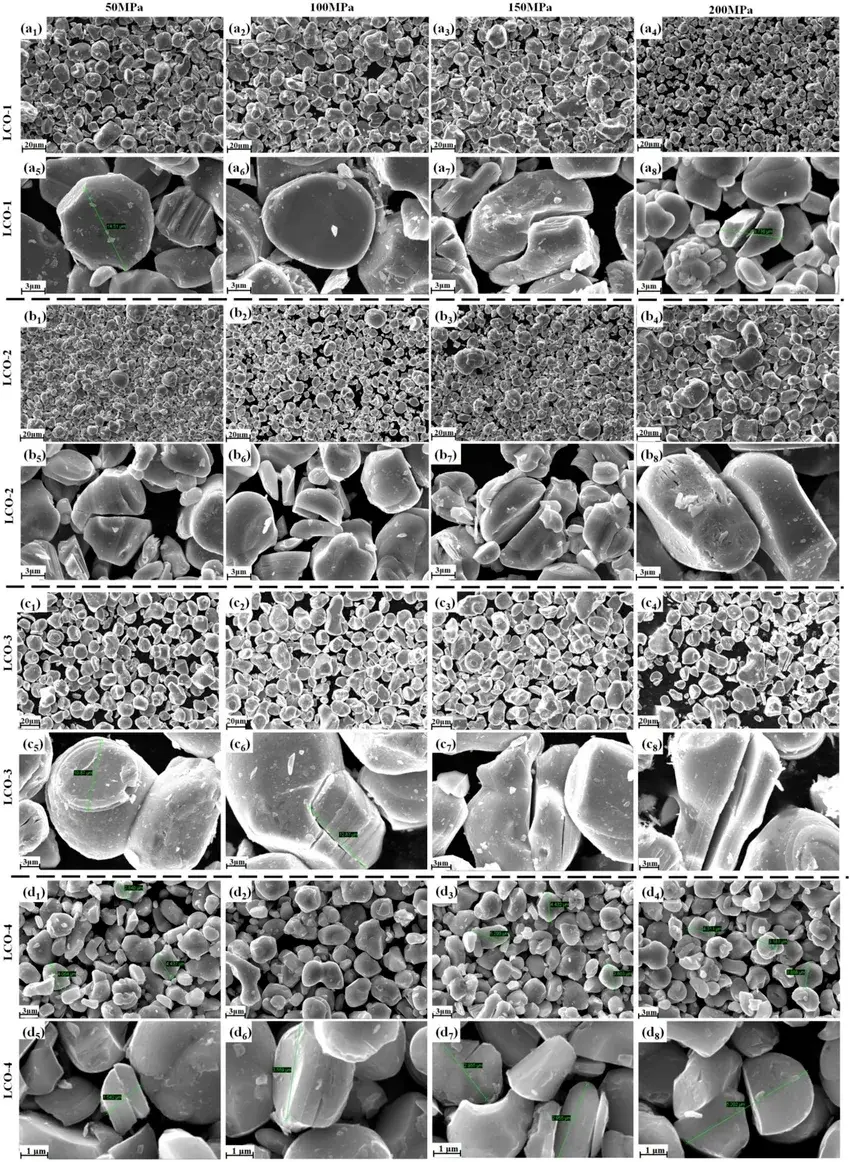
Figure 2. Low-magnification and high-magnification SEM images of four LCO powders at different pressures
The mechanical and electrical conductivity properties of the four LCO powders are shown in Figure. 3a-d. Compaction curves, including compaction density-pressure curves, compression cycle curves, stress-strain curves, and Heckel curves, are important tools for studying the filling, deformation, and fracture processes of powder materials under compaction. Figure 3a shows the axial stress and strain plots for four LCO samples. The magnitude of maximum and residual deformation is in the order of LCO-4> LCO-1> LCO-3> LCO-2, and the average particle size of LCO-4 is significantly smaller than that of the remaining samples. During the loading process, the powders were firstly rearranged, while the compressive stress did not change in the OQ stage. Secondly, in the QA stage, with the increase of compaction displacement, the compaction stress increased nonlinearly to the maximum stress point a. Finally, in the unloaded rebound AC stage, the closer the final C point was to B, the larger the plastic proportion of the powder was, in the order of LCO-4>LCO-1>LCO-3>LCO-2. The graphs of the compaction density versus the stress are shown in Figure. 3b. With the increase of axial stress, the compaction density of LCO powder increases continuously and finally reaches the ultimate compaction density at high enough stress. Among them, LCO-3 has the best compaction performance.
The porosity-pressure relationship is usually defined by Heckel’s equation, which is an empirical formula summarizing the relationship between compaction stress and density variation, expressed as:
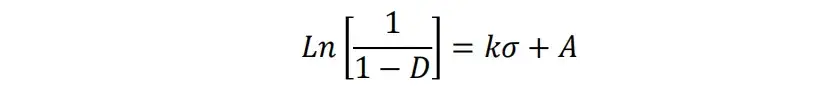
where σ is the axial compaction stress and D is the relative density of the powder under the corresponding pressure. k and A are the slope and intercept of the fitted straight line, representing the degree of plasticity and the state of the powder before deformation, respectively. the larger the value of k, the greater the change in density induced by the same change in stress, and the greater the plasticity of the powder. Experiments show that when k is certain, it indicates that the relative density change of the powder is caused by plastic deformation. If k is a variable, the fitting function is curved, indicating that the change in relative density is caused by rearrangement and fracture. The Heckel curves, fitted straight lines and differential curves for the lithium cobalt oxide(LCO) samples are plotted in Figure. 3c. k values are in the order of LCO-4 > LCO-1 > LCO-3 > LCO-2, i.e., the LCO-4 sample has the smallest average diameter and the largest deformation at the same pressure. In the combined comparison, the LCO-2 sample has the least plasticity. Figure 3d records the data of powder thickness and stress variation with time during compaction. As the load increases, the internal stress of the powder gradually increases, while the deformation gradually decreases and approaches the compacted thickness.The plastic deformation of LCO-4 is the largest, and the other samples have similar elastic and plastic properties.
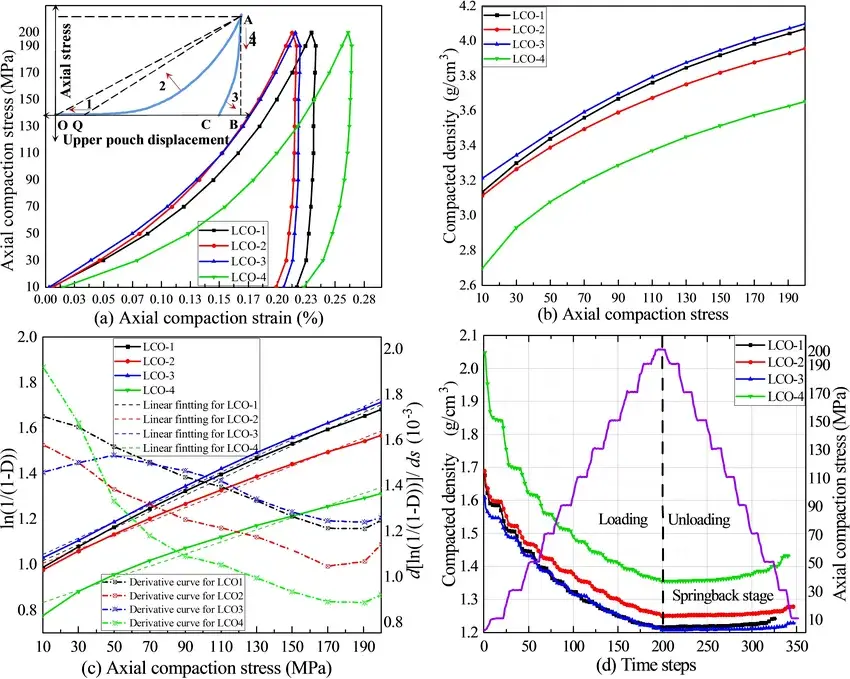
Figure. 3.(a) Plot of axial stress-strain during compaction; (b) Plot of compaction density and axial stress; (c) Plot of axial stress versus Heckel’s function/Heckel’s differentiation and fitting curves; and (d) Plot of compaction density and axial stress for different time steps.
Integration of the stress-strain curve can be used to calculate the work done by the powder to overcome the elastic and plastic deformation, respectively, as shown in Fig. 4a-d The smaller the average diameter, the smaller the plasticity ratio of LCO-4. For specimens with smaller average diameters, more work is required to achieve the same pressure, while the work done by spherical particles is mainly to overcome the friction and repulsion between particles in the particle rearrangement process, so that fracture is less likely to occur during compaction at various pressures.
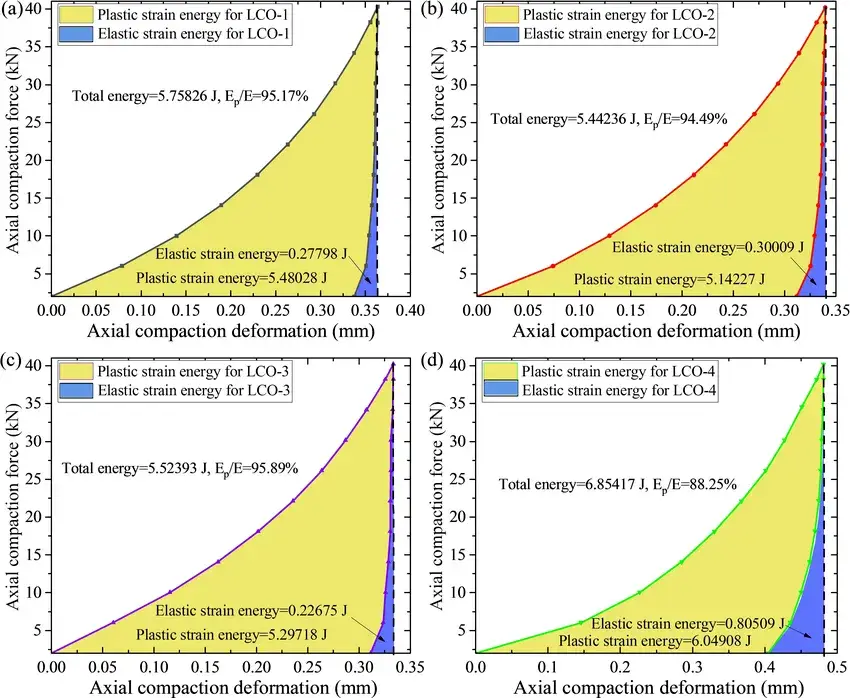
Figure. 4. Proportion of elastic deformation energy and plastic deformation energy during compression of four LCOs
Figures 5a-b show the changes in resistivity, conductivity and electrochemical properties during pressurization/unpressurization. The resistivity of LCO powder decreases with increasing pressure, and the order of conductivity is LCO-3 > LCO-2 > LCO- 4 > LCO-1. Figure. 6c shows the charging/discharging curves of LCO-4 samples at pressures of 50, 100, 150, and 200 MPa. In the first cycle, the LCO electrode at 200 MPa exhibited a higher capacity than the other samples due to the close contact between the LCO electrode particles. However, the LCO electrode at 200 MPa showed a large capacity decay after 200 cycles (Figure. 5 d). Excessive pressure leads to particle fragmentation, whereas the LCO electrode at 50 MPa, where the particle morphology remains relatively intact, maintains the highest capacity, as shown in Figure. 5d. Meanwhile, the Coulombic efficiency of all samples after cycling remained at ~100%, fully utilizing the active material. The experiments showed that appropriate pressure is favorable for good contact and cycling stability, but too much pressure will lead to reversible capacity decrease.
Figure. 5. Resistivity, conductivity and electrochemical properties of 4 LCO powders
4. Summary
The mechanical and electrochemical properties of lithium cobalt oxide powder were investigated by using the Powder Resistivity & Compaction Density Tester (PRCD3100) of IEST, and the stress-strain, compaction density and resistivity curves of lithium cobalt oxide powder were tested in real time. To a certain extent, the smaller the average diameter, the higher the axial deformation during compaction, the higher the resistivity and the higher the compaction density. This is because the smaller the diameter, the larger the specific surface area and the smaller the porosity, which cannot provide enough voids for mechanical deformation. The electrochemical performance of lithium cobalt oxide after different compaction is mainly affected by the difference in powder morphology, contact area and conductivity, indicating that the smaller the particle-integrated lithium cobalt oxide electrode is compacted, the better the cell performance.
5. Original Article
6. IEST Recommended Testing Instruments: Powder Resistivity & Compaction Density Measurement System(PRCD Series)
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.