-
iestinstrument
Research on Correlation Between Cathode Powder Resistance and Electrode Resistance
1. Preface
With the rapid development of the lithium battery industry, lithium-ion batteries have been widely used in mobile phones, computers, automobiles, energy storage and other fields. Users have higher and higher requirements for fast charging of the battery, and the corresponding requirements for the rate performance of the battery are also increasing. The rate performance of lithium-ion batteries is closely related to the battery resistance. The battery resistance includes ionic resistance and electronic resistance. Ion resistance mainly refers to the transmission resistance of lithium ions in the electrolyte in the electrode pores, the resistance of lithium ions through the SEI, lithium ions and electrons. The charge transfer resistance at the active material/SEI interface and the solid phase diffusion resistance of lithium ions inside the active material. The electronic resistance mainly refers to the positive and negative electrode active material powder resistance, current collector resistance, contact resistance between active materials, and contact resistance between active material and current collector and tab welding resistance, etc. In the actual battery research and development and production process, the ionic resistance part needs to be evaluated at the finished end of the battery, and the electronic resistance part can be quickly evaluated at the material and electrode piece. Therefore, the accurate evaluation of the material and electrode piece electronic resistance is of great significant for the finished battery cell.
In the full battery electronic resistance, the positive electrode resistance usually accounts for a relatively large amount. The positive electrode sheet resistance is affected by a variety of materials, including active materials, adhesives, and conductive carbon. The active material content ratio is as high as 95% or more. When the ratio of other materials is fixed, the powder resistance and the electrode sheet resistance are theoretically, there will be a certain degree of correlation. If this correlation can be determined, the evaluation of the cell resistance can be further advanced from the electrode end to the material end, which not only saves R&D and production costs, but also accelerates the R&D progress, which is beneficial to enterprises meet market demands faster and seize market opportunities. There are currently two methods for testing positive electrode powder resistance: the four-probe method and the two-probe method. The four-probe method is to characterize the lateral resistance of the surface of the powder tablet, and the two-probe method is to test the longitudinal resistance of the powder tablet. The method of testing the electrode resistance is usually the two-probe method. For details, please refer to the article “New method for monitoring stability and uniformity of battery electrode”. In order to ensure the comparability of the test results, the four-wire method based on the two-probe method and the double-disk electrode with controllable pressure were selected for the powder resistance and electrode resistance tests. The powder resistance tester reached a maximum applied pressure of 200MPa, it can make the compacted state of the powder closer to the compacted state of the powder in the electrode.
2. Experimental Equipment and Test Methods
2.1 Experimental Equipment
2.1.1 Powder resistance & compaction density test
Model PRCD1100 (IEST), electrode diameter 16mm, test pressure range 10~200MPa, holding time 10s. The equipment is shown in Figure 1(a) and 1(b).
Figure1. (a)PRCD1100 appearance: (b)PRCD1100 structure
1.1.2 Electrode resistance & compaction density test
Model BER1300 (IEST), electrode diameter 14mm, applied pressure 25MPa, holding time 25s. The equipment is shown in Figure 2(a) and 2(b).
Figure 2. (a)BER1300 appearance; (b)BER1300 structure
2.2 Test Method
2.2.1 Powder Resistance & Compaction Density Test
Take 1~2g of powder sample, weigh it and pour it into the fixture (PR-Device-16), pre-vibrate the powder on the pre-vibration instrument (Pre-V1) to make the accumulation state of the powder consistent before pressing. Put the fixture into the powder resistance meter PRCD1100, start the PRCDMS software to set the test pressure and pressure holding time parameters, and the software automatically reads the data of powder sample thickness, compacted density, resistance, resistivity, conductivity and other data.
2.2.2 Electrode Resistance & Compaction Density Test
Cut the rolled electrode into a rectangular size of about 5cm×10cm, place it between the two electrodes of the electrode resistance meter, start the MRMS software, set the parameters such as test pressure, holding time, active substance quality, etc., the software automatically reads Take data such as electrode thickness, compacted density, resistance, resistivity, conductivity, etc.
3. Correspondence Between Powder Resistance of Different Systems and Electrode Resistance
3.1 LCO System
Lithium cobalt oxide material is currently the most widely used cathode material for consumer batteries. Due to its large powder resistance and poor conductivity, lithium cobalt oxide material often needs to be modified, such as doping or coating. Figure 3(a) is the powder resistance test of the LCO material before and after modification, and the relationship curve between powder conductivity and powder compact density is automatically obtained.
From the figure, the compact density is less than 3.87g/cm3 (Pressure is applied at 75MPa), the electrical conductivity of the modified powder is less than that before modification, and when the compaction density is greater than 3.87g/cm3, the electrical conductivity of the modified powder begins to be superior to that before modification, and with the compaction density Increase rapidly. Comparing Figure 3(b) the conductivity of the electrodes (the compact density of the electrode 4.0g/cm3) prepared by the two powders with the same formula and process, the conductivity of the modified lithium cobaltate piece is significantly better than the modification previously, this proved to be effective for the modification of lithium cobaltate powder. The above results show that for this material, when the pressure state of the powder is consistent with the pressure state of the powder in the electrode, the trend that the powder resistance and the electrode resistance are consistent can be obtained.
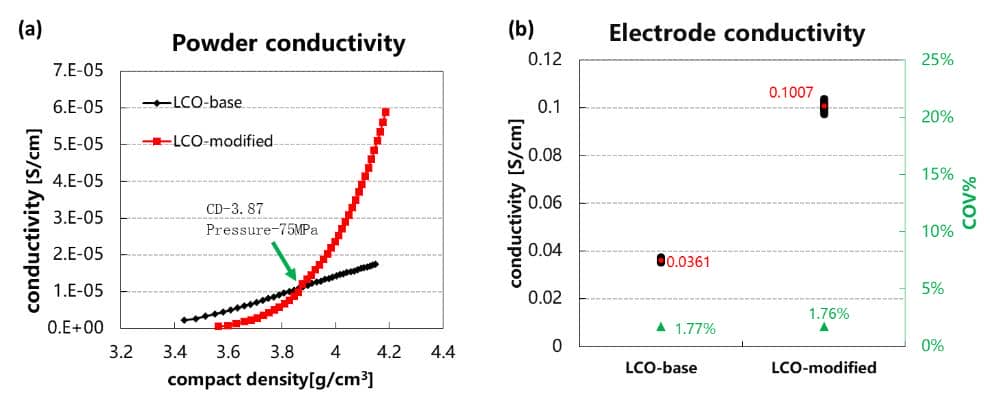
Figure 3 (a) Relation curve between conductivity and compacted density of lithium cobaltate powder before and after modification; (b) Comparison of conductivity of electrode piece prepared by lithium cobaltate material before and after modification.
3.2 Nickel Cobalt Manganese NCM System
Nickel-cobalt-manganese ternary materials are the first choice of cathode materials for most power batteries due to their higher gram capacity. The gram capacity of the ternary material is mainly affected by the Ni content. The higher the Ni content, the higher the reversible capacity, but on the other hand, with the increase of the Ni content, the battery cycle, rate and thermal stability will also have a certain degree influences. Figure 4(a) is the relationship between the powder conductivity of three different Ni contents and the powder compaction density. As can be seen from the figure, as the Ni content increases, the powder conductivity also shows an increasing trend. Comparing the electric conductivity of the electrode (the compact density of the electrode 3.2g/cm3) prepared with the same formula and process in Fig. 4(b), the trend of the electrode conductivity increasing with the increase of Ni content can also be obtained. The above results indicate that the ternary powder resistance and the electrode resistance have a corresponding correspondence.
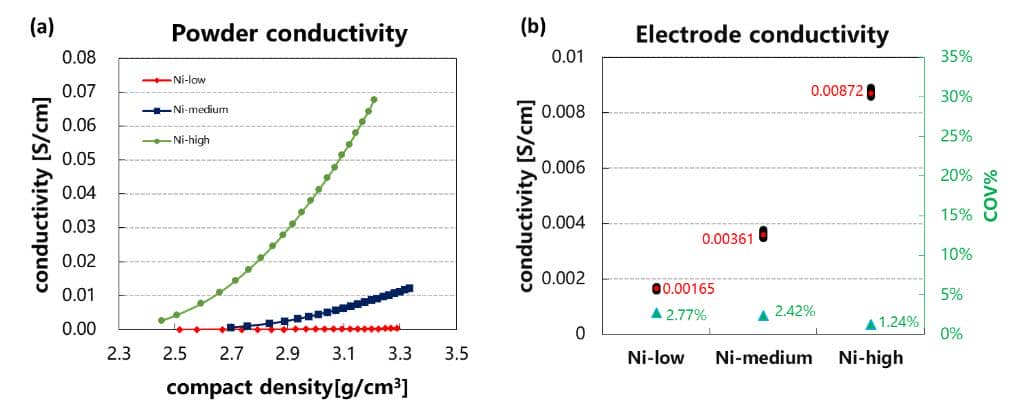
Figure 4 (a) Comparison of the relationship between the conductivity of the ternary powder before and after modification and the compacted density;
3.3 Lithium-ion Phosphate LFP System
With the market’s strong requirements for the safety of power batteries, lithium iron phosphate materials are gradually becoming the preferred cathode material for many power battery companies due to their high structural stability. However, the lithium iron phosphate material itself has poor conductivity and needs to be modified in advance before it can be used in power batteries that require high rate performance. Commonly used modification methods are: doping, carbon coating, and material nano7. Figure 5(a) is a comparison of the powder conductivity and compacted density of the LFP materials with three different modification methods. As shown in the figure, under the same compacted density conditions, the conductivity trends of the three materials are: LFP-1 >LFP-2>LFP-3. Comparing the conductivity of the electrode (the density of the electrode compacted at 2.4 g/cm3) prepared with the same formula and process, it can also be found that the electrode conductivity is: LFP-1>LFP-2> LFP-3 shows the same trend as powder, so the conductivity of powder can be used to quickly evaluate the effect of modification.
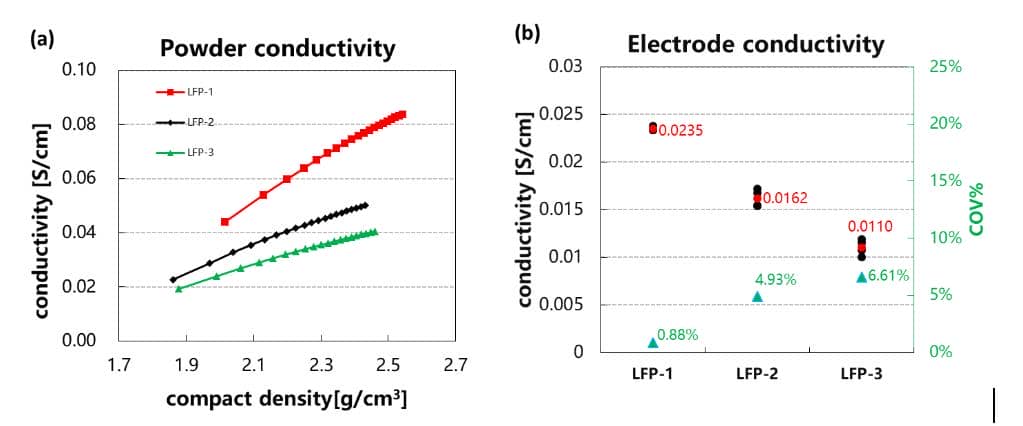
Figure 5 (a) Comparison of the relationship between the conductivity of the LFP powder before and after modification and the compacted density; (b) Comparison of the conductivity of the electrode prepared by the LFP material before and after modification
4. Conclusion
In this paper, the four-wire method and the controllable voltage double disc electrode method are used to test the powder resistance and electrode resistance of lithium cobalt oxide, ternary and lithium iron phosphate respectively, and the compact density of the two can be obtained simultaneously. When the compacted state of the powder is close to the compressed state of the powder in the electrode, the powder resistance of the three systems has the same trend as the electrode resistance, so the electronic resistance of the evaluation cell can be advanced from the electrode end to the powder end. Accelerating the R&D progress, saving R&D and production costs, is conducive to enterprises to meet market demand faster and seize market opportunities.
5. References
[1] B.G. Westphal et al. Influence of high intensive dry mixing and calendering on relative electrode resistivity determined via an advanced two point approach. Journal of Energy Storage 2017, 11, 76–85
[2] Hiroki Kondo et al. Influence of the Active Material on the Electronic Conductivity of the Positive Electrode in Lithium-Ion Batteries. Journal of The Electrochemical Society, 2019,166 (8) A1285-A1290
[3] Nils Mainusch et al. New Contact Probe and Method to Measure Electrical Resistances in Battery Electrodes Energy Technol. 2016, 4, 1550-1557
[4] Xu Jieru, Li Hong, et al., Conductivity measurement and analysis methods in lithium battery research Energy storage science and technology,2018,7(5) 926-955.
[5] Nie Lei, Qin Xing, Zhang Na, etc Research on pre-evaluation method of lithium ion battery resistance, power supply technology,2019, 43(4): 562-563;
[6] Zhuang Quanchao, Xu Shoudong, Qiu Xiangyun, etc Electrochemical impedance spectroscopy analysis of lithium ion batteries Chemical progress,2010,22(6):1044-1057;
[7] Yu Chenjie et al. Progress in Synthesis and Modification of LiFePO4 Cathode Material for Lithium Ion Rechargeable Batteries,2011,29(3): 468-470;
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.