-
iestinstrument
How to Evaluate Coating Uniformity on Current Collectors: A Resistivity-Based Method
1. Preface
Copper and aluminum foils serve as essential current collectors in lithium-ion battery electrodes, conducting electrons and supporting the active material layer. Their chemical and electrochemical stability significantly impacts cell performance, including cycle life, rate capability, and safety. An ideal current collector combines high conductivity, stability, strong adhesion, low cost, and flexibility 1.
2. Why Bottom-coat Uniformity Matters for the Current Collector
A thin, uneven carbon bottom coat can produce local zones of poor electrical contact or weak adhesion. These defects can increase local contact resistance, produce inhomogeneous current distribution, and ultimately accelerate capacity fade or cause failure under high-rate or long-term cycling. Consequently, manufacturers need simple, non-destructive metrics to monitor bottom-coat quality across rolls and after calendering. Thickness alone is not always diagnostic because a 2–3 µm coating can exhibit local thickness variations below the resolution of routine gauges. Instead, mapping the electrode sheet’s electrical response reveals functional uniformity that better predicts in-cell performance.This article demonstrates a practical method using electrode sheet resistance measurement to evaluate the uniformity of carbon-coated aluminum foil and its impact on final electrode properties.

Figure 1. Common surface-treated current collectors used in the lithium battery industry (Image source: network).
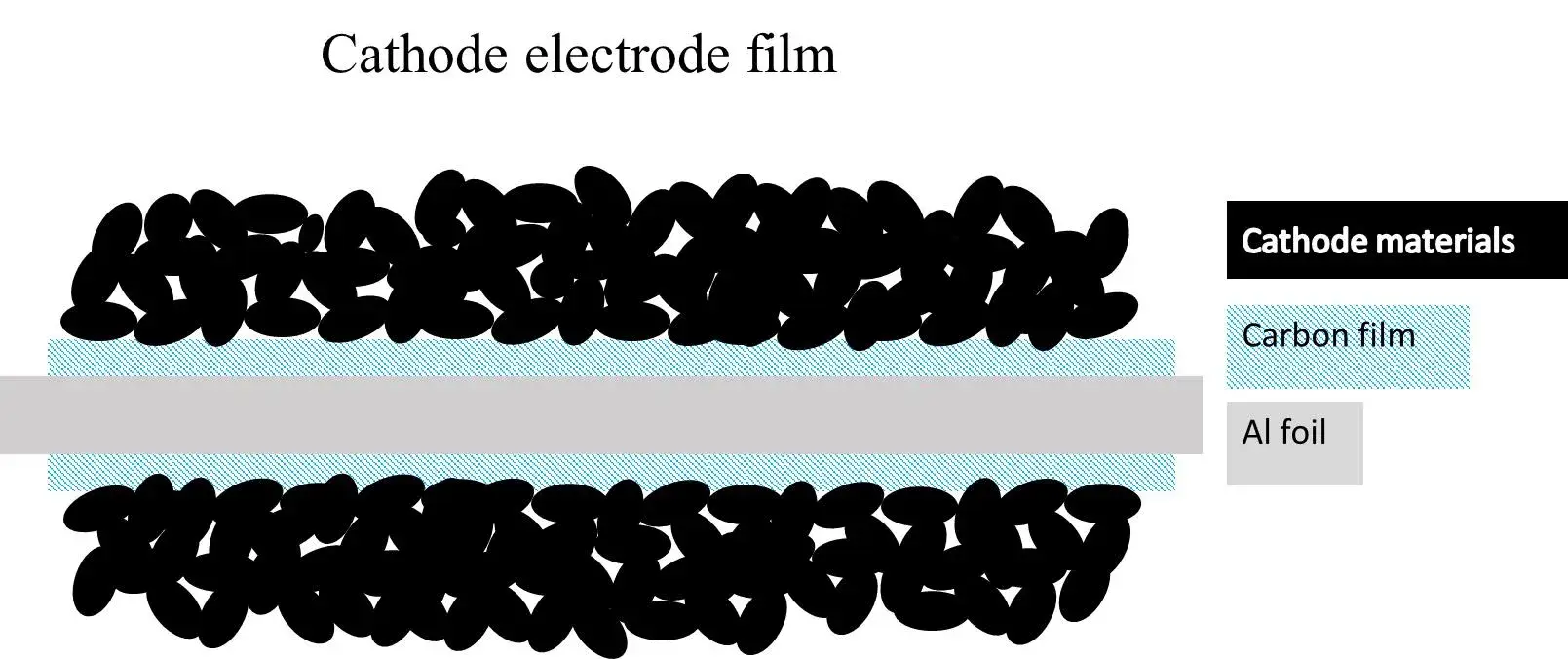
Figure 2. Schematic cross-section of an electrode with a primer-coated current collector.
3. Experimental Equipment and Test Methods
3.1 Experimental Equipment
Electrode resistance Tester, model BER Series (IEST), electrode diameter 14mm, can apply pressure 5-60MPa. The equipment is shown in Figure 3(a) and 1(b).
Figure 3. (a) BER2500 appearance diagram. (b) BER2500 structure diagram.
3.2 Four groups of samples to be tested
We compared four sample types to isolate the effect of the bottom coat:
-
Blank aluminum foil (no bottom coat),
-
Carbon-coated aluminum foil (bottom-coated current collector),
-
Blank electrode (Blank Al + active material coating), and
-
Carbon coated electrode (carbon-coated Al + active material coating).
3.3 Test Method
Samples were cut to ~5 cm × 10 cm rectangles and measured using a IEST Electrode Resistance Tester (BER1300). While the MRMS software records thickness, resistance, resistivity and conductivity automatically. For each material condition we recorded thickness statistics and resistance at a fixed test pressure; the testing sequence and platen pressure were kept identical across all four groups to ensure comparability.
4. Data Analysis: Resistivity Reveals Coating Inconsistencies
Thickness and resistivity results for the four sample groups are summarized in Figure 4.
4.1 Thickness Analysis
Measured thickness statistics indicate the carbon bottom coat on the aluminum foil is approximately 2.3 µm thick. Standard deviation (StDev) analysis of thickness across the four groups shows:
-
Blank Al foil ≈ Carbon-coated Al foil < Blank-foil electrode ≈ Carbon coated electrode
In other words, adding the thin carbon layer produced negligible change in overall foil thickness variation, and the finished electrodes (which include the active coating) show larger thickness variability. Because the bottom coat contributes only a few micrometers, thickness mapping has low sensitivity to coating uniformity; localized under- or over-coating can be masked by measurement noise or by the larger active-layer thickness.
4.2 Resistivity Analysis – The Key Metric
When we compared resistivity (Ω·m) across the four sample groups, the ordering was clear:
Blacnk Al foil < Carbon-coated Al foil < Blank electrode < Coated-coated electrode.
Several points emerge from these observations:
-
The carbon bottom coat increases the foil’s surface resistivity slightly versus bare aluminum, consistent with a thin, resistive carbon film atop a highly conductive metal substrate.
-
Adding the active coating (creating an electrode sheet) increases the apparent resistivity further due to the coating’s bulk resistance and the coating↔foil interface.
-
Importantly, the standard deviation of resistivity increases in the sequence Blank Al < Carbon-coated Al Foil < Blank-foil electrode < Carbon-coated electrode, indicating worse electrical uniformity after applying the bottom coat and then the active material layer.
This result suggests the carbon bottom coat—despite its benefits for adhesion—can be unevenly applied at sub-micron to micron scales; such unevenness becomes electrically visible once the electrode stack is completed. Local “misses” or excesses in the carbon film likely produce the larger resistivity spread observed in coated electrodes.
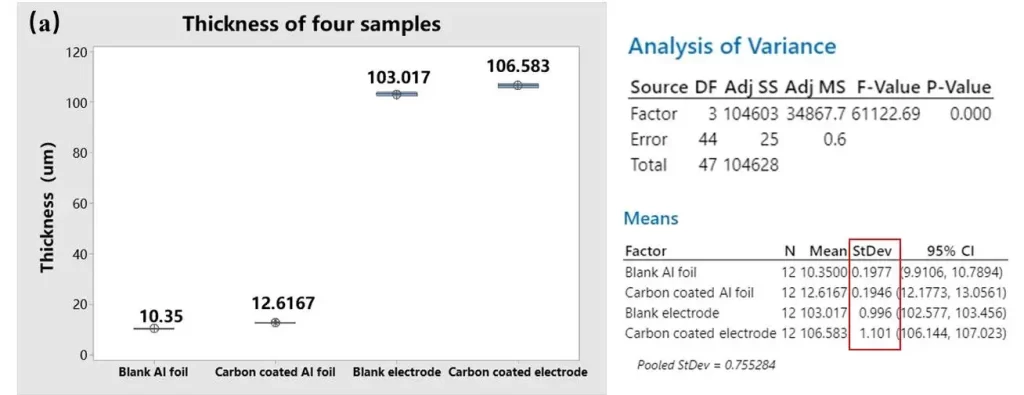
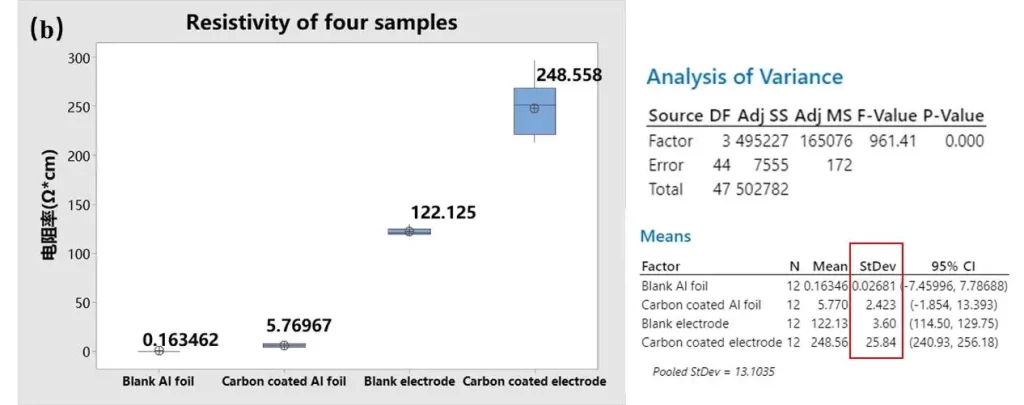
Figure 4. (a) Four sets of sample thickness data. (b) Four sets of sample resistivity data.
5. What BER-based Resistivity Mapping Reveals About Coating Quality
The BER1300’s resistance mapping captures functional uniformity rather than only geometric thickness. Since electrode performance depends on electrical continuity and contact integrity, resistivity mapping better predicts in-cell behavior:
-
Where bottom coat is too thin or absent, local contact resistance at the coating↔foil interface rises and manifests as higher measured resistivity.
-
Where bottom coat is overapplied or rough, local geometric inhomogeneity can also increase sheet resistance after coating and calendering.
-
Mapping across multiple punches provides a statistical picture (mean and StDev) that flags rolls or batches with excessive dispersion—useful for process control and quality assurance.
Therefore, resistivity distribution serves as a sensitive quality metric for carbon-coated aluminum foil and its downstream impact on electrode sheets.
6. Conclusion
Bottom-coat carbon layers on aluminum foils are thin (≈2–3 µm) and therefore difficult to assess by thickness measurement alone. IEST Electrode Resistance(BER Series) mapping provides a functional, sensitive method to evaluate carbon-coated aluminum foil uniformity and to quantify its effect on finished electrode resistivity. By tracking resistivity distributions (mean and StDev) across foil and finished sheets, manufacturers can better control bottom-coat processes, improve electrode uniformity, and reduce downstream cell variability and failure risk.
7. References
[1] Ni Jiangfeng, Zhou Henghui, etc. Research on current collectors for lithium-ion batteries[J]. Battery, 2005, 32(2): 128-130.
[2] Li Junpeng, Dang Haifeng, etc. Effect of aluminum current collector with surface treatment on performance of lithium-ion battery [J]. Electroplating and Finishing, 2005, 16(005).
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



