-
iestinstrument
Cyclic Voltammetry: Principles, Interface Effects and Practical CV Analysis
1. What is Cyclic Voltammetry?
Cyclic voltammetry (CV) is a cornerstone electrochemical technique that plots current versus applied potential while the electrode potential is swept cyclically. CV reveals oxidation and reduction peaks that reflect the redox behavior, kinetics and mass-transport properties of the electrochemical system. Because it is fast, sensitive and relatively simple, CV is widely used across battery research, electrocatalysis, sensors and materials screening. Even if you’re not an electrochemistry expert, you may have encountered a distinctive duck-shaped graph in journals, conferences, or equipment manufacturer websites. This characteristic pattern represents a Cyclic Voltammetry (CV) curve – one of the most fundamental techniques in electrochemical research. It looks like this:
Figure 1. Cyclic voltammogram
2. Fundamentals of Cyclic Voltammetry: Oxidation and Reduction Mechanisms
Cyclic voltammetry enables researchers to study electron transfer processes through controlled voltage application. Unlike traditional chemistry requiring chemical reductants, electrochemical reduction occurs via direct electron transfer to electrodes.
The technique involves applying a linearly changing voltage to an electrode while measuring current response. The potential scans from an initial value to a set limit then returns to the starting point, completing one cycle. This cyclic voltammetry analysis reveals:
-
Oxidation peaks during forward scanning (low to high voltage)
-
Reduction peaks during reverse scanning
-
Specific potentials where redox reactions occur
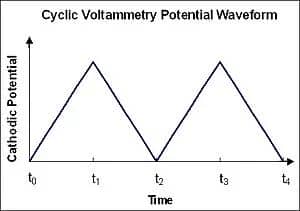
Figure 2. Applied potential curve
3. Why the Electrode–Electrolyte Interface Matters
The electrode–electrolyte interface is a complex, multiscale environment where electron transfer, ion transport and surface chemistry intersect. Key factors that influence CV behavior include:
-
Electrode material and morphology: surface area, crystal faces, defects and porosity change available active sites and double-layer capacitance.
-
Electrolyte composition: solvent, salt, additives and ion concentration affect mass transport and reaction pathways.
-
Interfacial films: SEI layers, oxide films or adsorbed species alter charge-transfer resistance and can shift or suppress peaks.
-
Scan rate and transport regime: faster scan rates reduce diffusion-layer thickness and amplify kinetic effects, often increasing peak currents and changing peak shapes.
Understanding these variables is essential when interpreting cyclic voltammograms for material evaluation or device diagnostics.
4.Cyclic Voltammetry Instruments — From Bench to Multi-Channel Systems
A reliable cyclic voltammetry instrument is key to high-quality measurements. Modern CV-capable analyzers integrate potential control, current measurement, data acquisition and safety limits. Instruments range from single-channel potentiostats for laboratory R&D to multi-channel systems for parallel screening. For battery and electrode research, look for features such as wide potential windows, high current capability, configurable scan rates, and software that supports automated CV sequences and data export.
5. Advanced Cyclic Voltammetry Instrumentation: ERT7008 Electrochemical Analyzer
Modern cyclic voltammetry instruments like the IEST ERT7008 series provide comprehensive testing capabilities integrating CV, EIS, and charge-discharge functions. Key features include:
-
Eight-channel parallel testing capability
-
Customizable scan rates (0.1 mV/s to 10 V/s)
-
Wide voltage range (±10 V)
-
High-precision current measurement (nA to A)
-
Automated parameter control and data acquisition
Figure 3. IEST ERT Series
The IEST ERT7008 series meets the requirements of conventional electrochemical testing. The specific parameters are as follows:
| ERT6008(Cyclic Voltammetry Analyzer) | ERT7008(Electrochemical Impedance Analyzer) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Photo |  |
 |
|||||||||
| Type | ERT6008-5V100mA | ERT6008-5V6A | ERT6008-5V12A | ERT7008-5V100mA | ERT7008-5V6A | ERT7008-5V12A | |||||
| Voltage | ±5V | ±5V | ±5V | ±5V | |||||||
| Current | 10nA~0.1mA 0.1mA~1mA 1mA~10mA 10mA~100mA |
0.6μA~6mA 6mA~60mA 60mA~0.6A 0.6A~6A |
1.2μA~12mA 12mA~120mA 120mA~1.2A 1.2A~12A |
10nA~0.1mA 0.1mA~1mA 1mA~10mA 10mA~100mA |
0.6μA~6mA 6mA~60mA 60mA~0.6A 0.6mA~6A |
1.2μA~12mA 12mA~120mA 120mA~1.2 A 1.2A~12A |
|||||
| ★EIS testing ★ | × | × | × | EIS (Electrochemical Impedance Spectroscopy) testing: 0.01Hz~100kHz |
|||||||
| ★Electrochemical features ★ |
Cyclic Voltammetry (CV), Linear Sweep Voltammetry (LSV), GITT/PITT/CA/CP Testing | Cyclic Voltammetry (CV), Linear Sweep Voltammetry (LSV), GITT/PITT/CA/CP Testing | |||||||||
| Temp. Chamber | 10~80℃ | -20~80℃ | -20~80℃ | ||||||||
| Channel | 8 | 8 | |||||||||
| Maximum Sampling Rate | 100 SPS | 100 SPS | |||||||||
| Output | Four-Electrode Aviation Connector (Supports three-electrode testing with auxiliary voltage integration) |
Four-Electrode Aviation Connector (Supports three-electrode testing with auxiliary voltage integration) |
|||||||||
| Current & Voltage Control Accuracy | ± 0.01% F.S. | ± 0.01% F.S. | |||||||||
| CP&CR Accuracy | ± 0.02% F.S. | ± 0.02% F.S. | |||||||||
| Resolution | 16bit | 16bit | |||||||||
| Input Power | 30W | 500W/1000W | 30W | 500W/1000W | |||||||
| Operating Modes |
Charge-Discharge Modes: Constant Current Charge/Discharge (CC) Constant Voltage Charge/Discharge (CV) Constant Current-Constant Voltage Charge/Discharge (CC-CV) Constant Power Charge/Discharge (CP) Constant Resistance Charge/Discharge (CR) DCIR (Direct Current Internal Resistance) Rate Charge/Discharge Electrochemical Modes: |
||||||||||
5. Practical Applications and Cyclic Voltammetry Analysis
5.1 Parameter Optimization
When designing cyclic voltammetry experiments, carefully select and document these parameters:
-
Potential window (initial & final potentials): set to encompass expected redox events but avoid decomposition.
-
Scan rate: common ranges span mV·s⁻¹ to V·s⁻¹; use multiple rates to probe kinetics vs diffusion.
-
Number of cycles & equilibration: allow stabilization (conditioning) cycles and monitor changes across cycles.
-
Data resolution (points per cycle): higher resolution improves peak definition but increases data size.
-
Current thresholds & safety limits: protect electrodes and cells, especially for battery materials.
Combine CV with complementary techniques (EIS, chronoamperometry, in-situ spectroscopy) to disentangle overlapping processes. Once these parameters are set, the testing can commence. After testing, you will obtain your own cyclic voltammogram. If all goes well, you may get a curve similar to the following:
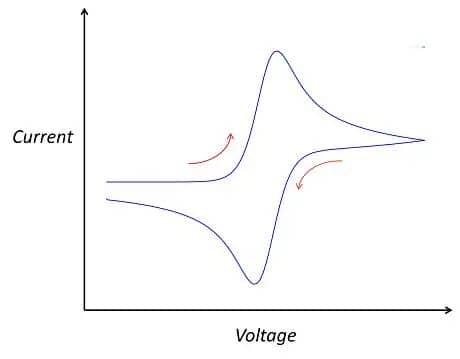
Figure 4. The standard CV curve schematic
5.2 Scan Rate Effects
As shown in Figure 5, scan rate dramatically influences voltammogram morphology:
-
Faster scans produce higher peak currents
-
Slower scans provide better resolution of closely spaced peaks
-
Scan rate studies reveal reaction kinetics and diffusion characteristics
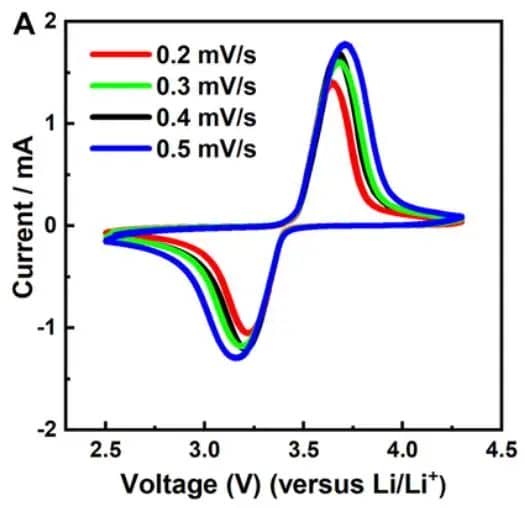
Figure 5. The cyclic voltammograms of LFP material at different scan rates.
5.3 Qualitative and Quantitative Analysis
While primarily used for qualitative assessment, cyclic voltammetry analysis enables quantitative measurement of:
-
Diffusion coefficients
-
Electron transfer rate constants
-
Activation energies
-
Concentration of active species
-
Surface coverage of adsorbed molecules
The technique’s sensitivity to time-scale variations allows researchers to observe different phenomena by adjusting scan rates, effectively “zooming” in and out of electrochemical processes.
6. Common Applications in Battery Research
In battery and electrode development, cyclic voltammetry is used to:
-
Screen new electrode materials and additives.
-
Identify redox-active impurities or side reactions.
-
Assess reversibility and kinetics of lithium insertion/extraction.
-
Guide electrolyte formulation by revealing stability windows and decomposition.
CV remains an indispensable first-line test for material characterization and troubleshooting.
7. Conclusion
Cyclic voltammetry represents a powerful, sensitive electrochemical characterization technique with broad applications in battery research, fuel cell development, electrocatalysis, and sensor design. High-quality cyclic voltammetry analysis requires proper instrument selection, careful control of test parameters, and an understanding of interface effects and transport regimes. When interpreted correctly and combined with complementary diagnostics, CV accelerates materials discovery and improves device reliability.
The IEST ERT7008 series cyclic voltammetry instruments provide researchers with robust tools for comprehensive electrochemical characterization, enabling advances in material development and system optimization across numerous applications.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



