-
iestinstrument
Study on the Correlation of Slurry Resistivity and Formulation
1. Preface
A lithium-ion battery electrode slurry is a critical solid-liquid mixture consisting of active material, conductive additive, and binder dispersed in a solvent. Achieving an optimal battery slurry formulation and slurry resistivity is not trivial; it requires meeting specific criteria: excellent dispersion of all components, the opening of chain-like conductive additives, an ideal spatial arrangement of materials, and a stable suspension that prevents settling. The uniformity and stability of the slurry directly dictate cell-to-cell consistency and overall electrochemical performance. Poor dispersion or rapid settling prevents the formation of a continuous electron-conducting network, severely limiting the electrode’s capability.
In electrode design, the conductive additive forms a 3D network that bridges active material particles, serving as the essential highway for electron transport. This is particularly vital for cathode materials, which often possess low intrinsic electronic conductivity. Consequently, selecting the right conductive additive—considering its type, morphology, particle size, addition sequence, and dosage—is a key lever for tuning battery properties like rate performance and cycle life. The distribution state of the conductive additive is equally crucial. As Figure 1 illustrates, it can exist in non-ideal states (agglomerated or isolated) or the ideal state: uniformly dispersed, coating active particles and forming a connected, percolating network that ensures every particle is electrically accessible.
Measuring slurry resistivity provides a direct, quantitative method to assess dispersion quality and conductive network formation at the slurry stage itself. While conductive network modeling is common, using slurry resistivity as a practical performance metric is less explored. This study systematically varies solid content to analyze its relationship with slurry resistivity and battery slurry viscosity. Furthermore, we employ current-voltage (I-V) testing to confirm the dominant conduction mechanism within the slurry.
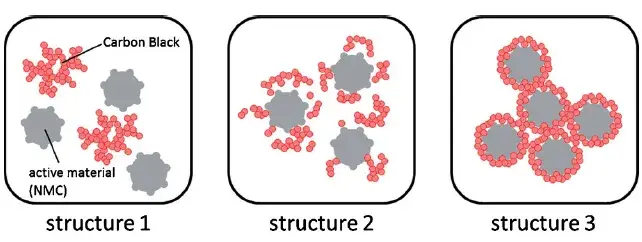
Figure 1. Distribution status of the conductive agent in the slurry
2. Experimental Approach and Instrument
2.1 Test Equipment
The IEST Lithium Battery Slurry Resistivity Tester(BSR2300)(Figure 2) was used to characterize the slurry resistivity of cathode and anode formulations with varying solid content and viscosity.
Figure 2. IEST BSR2300 Appearance Diagram
2.2 Slurry Ratio
Slurries were prepared according to the battery slurry formulation detailed in Table 1
Table 1. Slurry ratios
3. Results & Discussion
3.1 The Solid Content Trade-off: Viscosity vs. Resistivity
According to the formula in Table 1, the anode and cathode electrode slurries with different solid contents were configured and tested for viscosity and resistivity, respectively, as shown in Table 2, both anode and cathode electrode slurries showed increasing viscosity and decreasing resistivity with the increase of solid content.
When the solid content of LCO is greater than 50%, the viscosity increases sharply, which may be due to the increasing proportion of lithium cobaltate particles per unit volume as the solid content increases, and the collision of large-density lithium cobaltate particles exacerbates the interaction force between the particles in the system, which leads to an increase in the viscosity, and the point of sharp increase is the threshold point of the sharp change in the force between the particles. When the graphite solid content of 35% or less, with the increase of solid content resistivity accelerated decrease, when the solid content is greater than 35%, the slurry resistivity with the solid content change is slower, this is because as the solid content increases, more and more particles to build an effective conductive network, when the conductivity is reached after the threshold is not a significant increase in conductivity.
Table 2. Anode and Cathode Electrode Slurry Resistivity & Solid Content & Viscosity
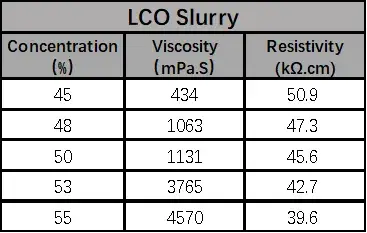
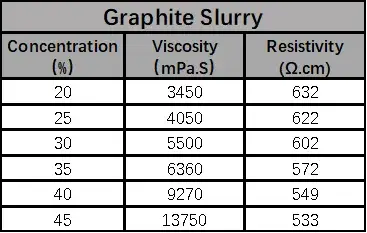
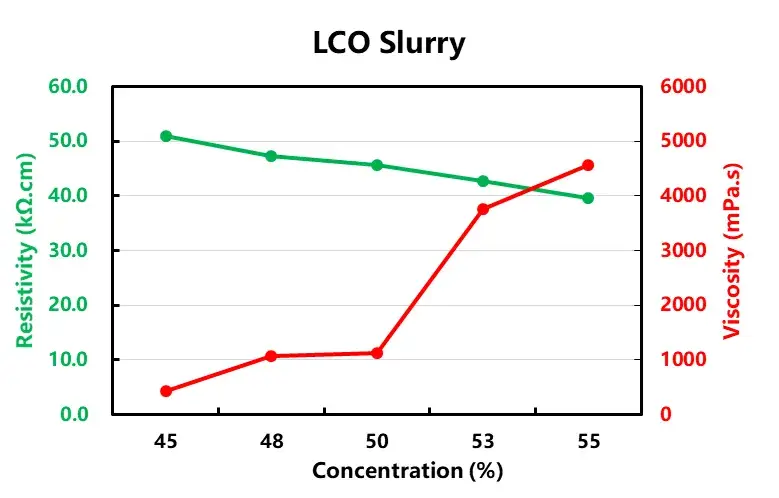
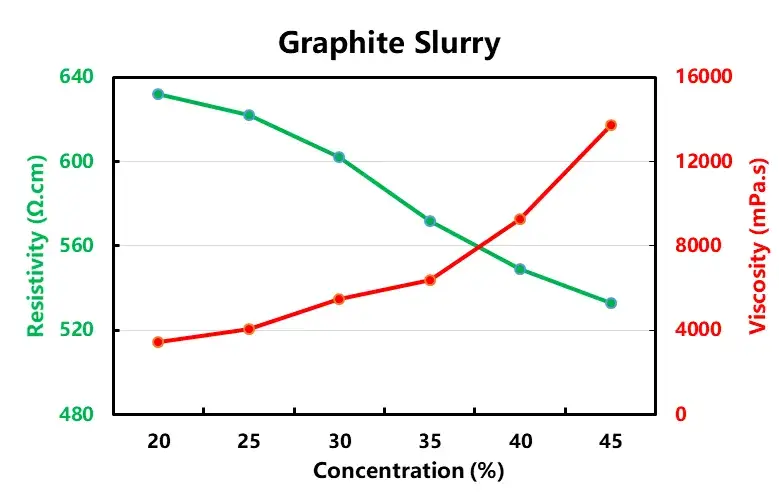
Figure 3. Trend of resistivity and viscosity of anode and cathode electrode slurry with solid content
3.2 Verifying the Conduction Mechanism
In order to further investigate the conductive mode in the lithium positive and negative electrode slurry system, I-V experiments were designed to verify it, as shown in Figure 4. For LCO slurry and graphite slurry, 0.001mA, 0.0015mA, 0.002mA, 0.0025mA, 0.003mA current is applied respectively, and the voltage signal is collected. Figure 4 shows that the current-voltage linear relationship is obvious and basically composite Ohm’s law, indicating that the conductive type of lithium cobalt oxide cathode slurry and graphite anode slurry is mainly dominated by electron conductivity, i.e., the electrons are conducted to the particles themselves through the contact between the particles, which in turn builds a multidimensional conductive network and exhibits a certain degree of conductivity.
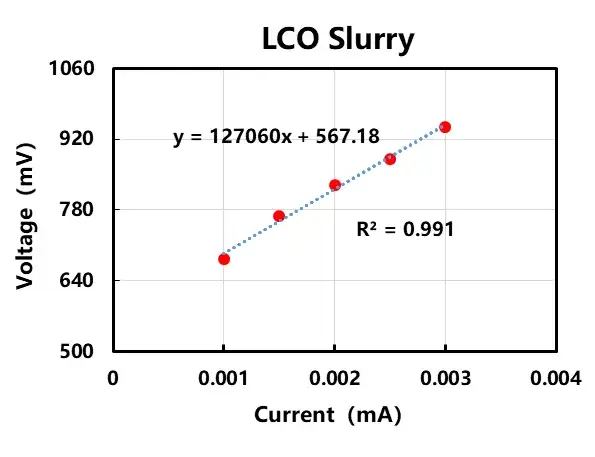
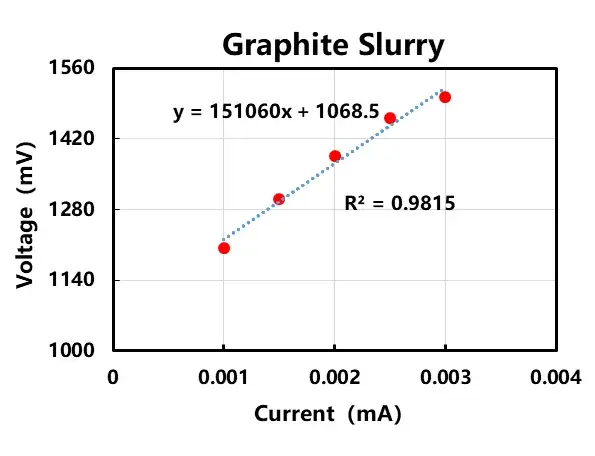
Figure 4. I-V curves for anode and cathode electrode slurry
4. Summary
In this paper, BSR2300 (IEST) was used to analyze the relationship between the slurry resistivity, viscosity and solid content of lithium battery anode and cathode electrode slurry, and found that the anode and cathode electrode slurry resistivity is significantly reduced with the increase of solid content. Meanwhile, coupled with the identified viscosity thresholds, provides actionable guidance for process engineers. By monitoring slurry resistivity, manufacturers can objectively assess dispersion quality and predict the effectiveness of the conductive network before coating and drying, enabling faster optimization cycles and more consistent, high-performance battery electrodes.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.