-
iestinstrument
Cyclic Voltammetry Analysis: A Practical Guide for Electrode Material Characterization
1. Understanding Cyclic Voltammetry Principle
Cyclic Voltammetry (CV) is a fundamental electrochemical characterization technique, often described as a “health check” for electrode materials. The core cyclic voltammetry principle involves applying a triangular, linearly changing potential waveform (e.g., scanning from -0.2V → 1.0V → -0.2V) to the working electrode while simultaneously measuring the current response. This generates a closed current-voltage loop known as a CV curve, effectively mimicking the dynamic charge-discharge behavior of a battery.
A well-interpreted CV curve reveals critical material properties:
-
Redox Characteristics: Peak potentials indicate the reaction voltage of active materials, while peak currents reflect reaction kinetics.
-
Reversibility: A small potential difference (ΔEp) between oxidation and reduction peaks signifies high electrochemical reversibility.
-
Mass Transport Mechanism: Analyzing the dependence of the current on scan rate helps distinguish between diffusion-controlled and surface-controlled processes.
-
Stability: Changes in peak current or area over multiple cycles quantify material degradation.
In lithium-ion battery research, CV clearly visualizes processes like Li⁺ intercalation/de-intercalation (e.g., ~0.2V vs. Li⁺/Li for graphite) or electrolyte decomposition (>4.5V), functioning much like an “electrochemical EKG.”
2. Experimental Case Study & Data Interpretation
To demonstrate practical cyclic voltammetry analysis, we tested a standard 24 mAh coin cell (LiCoO₂ cathode vs. graphite anode) using the IEST ERT6008-5V100mA High-Precision Electrochemical Analyzer (accuracy: 0.01% F.S.). Tests were conducted at scan rates of 0.1, 0.2, and 0.5 mV/s within a voltage range of 3.0-4.2V vs. Li⁺/Li.
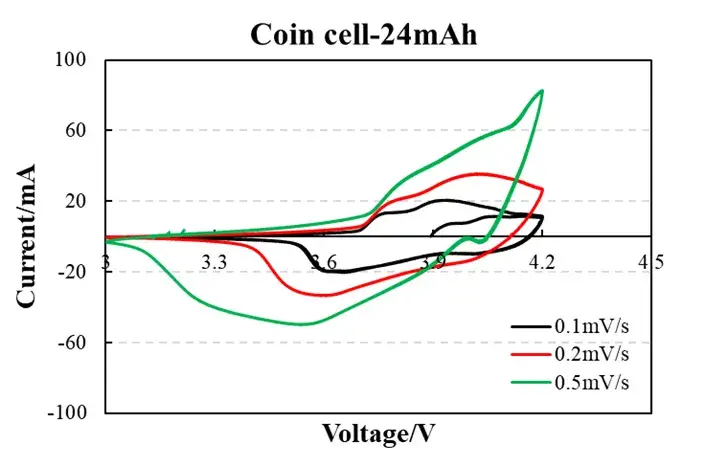
Figure 1. Experimental Data
2.1 Analysis of CV Curves at Different Scan Rates
At a low scan rate (0.1 mV/s, black curve), the peak current is low (~0.5 mA), and the oxidation/reduction peaks show good symmetry, indicating minimal polarization and a diffusion-controlled reaction. The peak separation ΔEp ≈ 60 mV is close to the theoretical value (59 mV/n for a one-electron process), confirming highly reversible Li⁺ intercalation/de-intercalation.
At a high scan rate (0.5 mV/s, green curve), the peak current increases to ~1.2 mA, but ΔEP widens to 90 mV. This reflects increased charge-transfer resistance and the onset of kinetic polarization. The preservation of the curve shape suggests excellent structural stability with no parasitic side reactions.
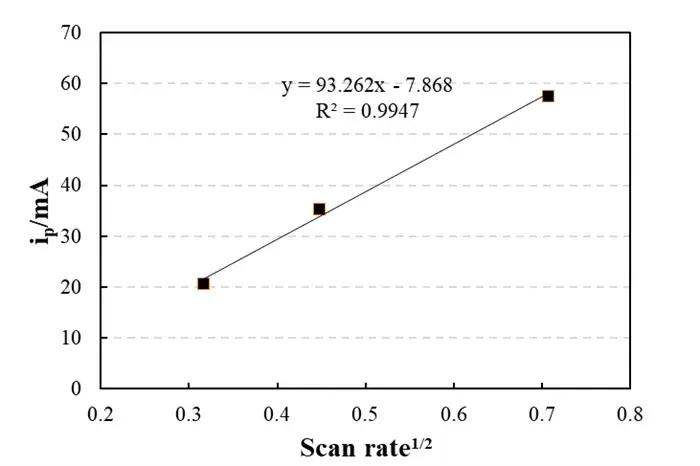
Figure 2. Linear Fitting Plot
2.2 Validating the Diffusion-Controlled Mechanism
A key step in cyclic voltammetry analysis is verifying the reaction mechanism. Plotting the peak current (i_p) against the square root of the scan rate (√v) yielded a linear relationship (R² = 0.9947), described by the equation: y = 93.262x – 7.868. This strong linearity perfectly aligns with the Randles-Ševčík equation, confirming a lithium-ion diffusion-controlled process.
The slope of 93.262 can be used to calculate the Li⁺ diffusion coefficient (D), resulting in a value on the order of 10⁻¹⁰ cm²/s, which is typical for graphite materials. The intercept (-7.868 mA) is close to zero, indicating negligible contribution from non-Faradaic (double-layer charging) currents.
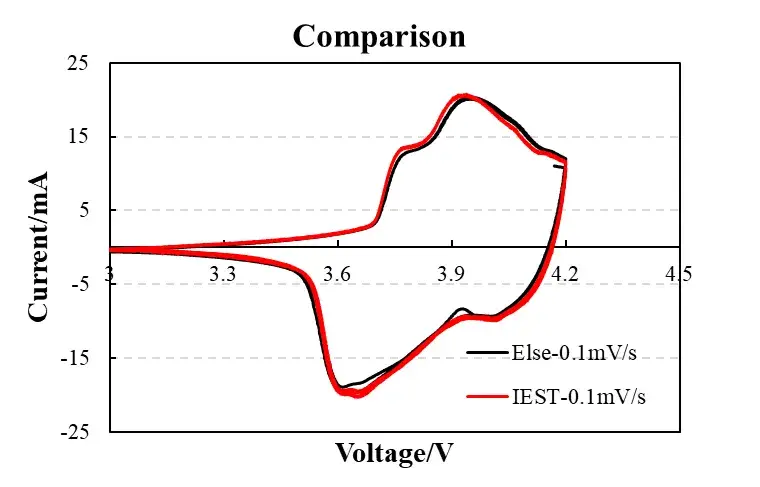
Figure 3. Device comparison (IEST vs. commercial workstation)
2.3 Instrument Performance Validation
The CV curves obtained from the IEST High precision Battery Test Equipment were compared against data from a commercial electrochemical workstation (e.g., BioLogic). The curves showed an overlap of >95%, with no anomalous fluctuations, particularly in the high-potential region (4.0-4.2V). This demonstrates that the data quality and fidelity of the IEST instrument meet rigorous research-grade requirements.
3. Key Equation & Technical Summary
The experimental results are governed by the Randles-Ševčík equation, which is central to quantitative cyclic voltammetry analysis for diffusion-controlled systems:
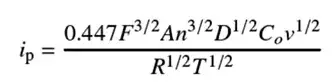
Figure 3. Randles–Ševčík equation
Variables:
-
i_p: Peak current(A)
-
F: Faraday constant
-
A: Electrode area (cm²)
-
n: Number of electrons transferred
-
D: Diffusion coefficient (cm²/s)
-
C: Concentration (mol/cm³)
-
v: Scan rate (V/s)
-
R, T: Gas constant & absolute temperature
In essence, this equation demonstrates that the peak current ipip is directly proportional to the square root of the scan rate vv. Consequently, while the shape of the CV curve varies with scan rate, its fundamental profile remains consistent.
4. Practical Data-quality Notes — Instrument Performance and Benchmarking
The study compared CV traces acquired on the IEST ERT6008 with a commercial electrochemical workstation (BioLogic). Agreement between the two systems exceeded 95% overlap in curve shape; deviations were ≤ 1.5% overall, and no anomalous features appeared in the high-potential region (4.0–4.2 V). These comparisons demonstrate that a well-specified benchtop instrument with appropriate calibration can deliver research-grade CV data for diagnostic and materials-screening workflows.
Instrument selection checklist:
-
Verify absolute voltage and current accuracy (calibration specs such as 0.01% F.S. are valuable for small-current coin-cell work).
-
Confirm ADC resolution, sampling rate and low-noise front-end for reliable peak-shape fidelity at low scan rates.
-
Record and publish environmental and cell-assembly metadata (temperature, cell geometry, reference electrode details where applicable).
5. CV Best Practices and Common Pitfalls in Cyclic Voltammetry Analysis
-
Choose scan-rate range rationally. Very low scan rates approach steady-state but take long to measure; very high rates emphasize kinetics but can conflate capacitive currents with faradaic peaks. Use a set of rates that span at least one order of magnitude for robust regression (as in this study: 0.1–0.5 mV·s⁻¹).
-
Subtract background (non-faradaic) current where necessary before peak fitting; this improves peak-area and peak-height accuracy.
-
Fit peaks consistently. Use the same baseline and smoothing method across datasets; report smoothing parameters if used.
-
Report ΔEp and i_p vs √ν together — they jointly indicate whether a process is kinetically controlled, diffusion-limited, or complicated by pseudocapacitive/surface phenomena.
-
Correlate CV with complementary tests (GITT, EIS, galvanostatic cycling) to separate diffusion limits from interfacial charge-transfer limitations.
6. Summary
This case study showcases the power of Cyclic Voltammetry as an indispensable diagnostic tool for electrode materials and highlighting the exceptional performance of IEST instrument. Our equipment maintains data accuracy consistent with internationally renowned brands (e.g., BioLogic), with comparative deviations rigorously controlled within 1.5%, coupled with significant cost advantages.
More notably, we will launch a revolutionary intelligent electrochemical data analysis system in Q2 2025, enabling advanced CV curve processing:
-
Batch Cyclic Voltammetry(CV) curve visualization
-
Feature point identification and smoothing algorithms
-
Automated peak recognition/fitting for extracting peak position, height, and area parameters
-
Cyclic Voltammetry(CV) curve smoothing functionality
Innovatively, we have integrated cloud-based data synchronization, enabling real-time interfacing between central and cloud databases.
With its precision, intelligence, and reliability, IEST electrochemical analyzers are becoming the primary electrochemical diagnostic platform in growing numbers of laboratories. We cordially invite you to experience this professional solution delivering comprehensive testing capabilities from R&D through mass production.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



