-
iestinstrument
Impact of Pressure on Separator Ionic Conductivity in Lithium-Ion Batteries
Abstract
Separator ionic conductivity controls Li⁺ transport between electrodes and therefore strongly affects power, rate capability and ageing in lithium-ion cells. This study uses IEST’s multi-channel EIC2400M tester to quantify how applied through-thickness pressure (0–3 MPa range) alters the ionic conductivity of two commercial separator samples. Results show a roughly linear decline in ionic conductivity with increasing compressive pressure; the magnitude of decline depends on separator microstructure and porosity. We also present the ionic conductivity calculation method used and practical recommendations for separator selection and cell mechanical design.
1. Introduction — why separator ionic conductivity matters
The intercalation/deintercalation or deposition/stripping of lithium ions, along with side reactions such as continuous growth of the SEI film and gas generation, can induce internal pressure in batteries. This pressure influences various battery performance characteristics through interfacial effects[1]. Due to the porous structure and material properties of separators, significant deformation occurs under pressure, leading to pressure-dependent changes in separator ionic conductivity. Studies have shown that the deformation-pressure relationship curve of porous separators during compression can be divided into three stages: elastic (Ⅰ), plastic (Ⅱ), and densification (Ⅲ) stages, as illustrated in Figure 1.
-
Elastic Stage (Ⅰ): Reversible deformation with maintained ionic conductivity.
-
Plastic Stage (Ⅱ): Irreversible pore collapse and reduced ion transport capability.
-
Densification Stage (Ⅲ): Full pore closure and loss of ionic conductivity[2-3].
In Stage Ⅰ, the porous separator undergoes substantial elastic deformation under pressure but rapidly restores its original morphology upon pressure release, maintaining unchanged ion transport properties. During Stage Ⅱ, the separator experiences plastic compression, where the pore structure becomes irreversibly deformed. Ion transport capability cannot be fully preserved, and the separator fails to fully recover its initial state after pressure removal. In Stage Ⅲ, the porous structure collapses and densifies, with pores completely closing to form a compact structure. At this stage, the separator loses its ion transport functionality[2-3].
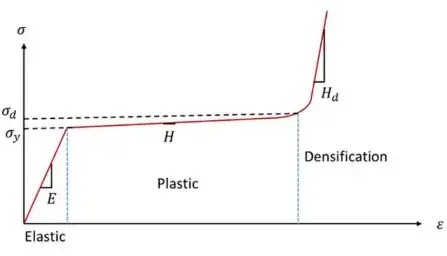
Figure 1. Fatigue-strain relationship diagram of discomfort during compression[2]
Data show that the membrane deformation is 0.5 at 0-20 MPa, and increases from 0.5 to 0.55 at 20-30 MPa. At 0-12 MPa, the ionic conductivity of the membrane decreases with the increase of stress, and the two are linearly related. When the stress is higher than 12 MPa, the separator ionic conductivity decreases rapidly and gradually tends to be stable [4]. The different separator ionic conductivity decays differently with the increase of pressure. This paper tests the changes in the two separator ionic conductivity at 0-3 MPa to explore the effect of pressure on the separator ionic conductivity.
2. Experimental setup & ionic conductivity calculation
2.1 Test Instrument
The Multi-channel Separator Ionic Conductivity Test System (EIC2400M) developed by IEST is used as shown in Figure 2. The equipment contains 4 test channels and can provide a high-purity argon atmosphere to achieve electrochemical impedance spectroscopy testing of multi-channel symmetrical batteries. The pressure range is 10~50Kg and the frequency range is 100KHz~0.01Hz.
Figure 2. IEST Electrode Tortuosity Tester & Separator Ion Conductivity Tester(EIC Series)
2.2 Test Samples
Two separator variants (hereafter Separator A and Separator B) were tested. To extract through-thickness impedance consistently, each separator was measured as 1, 2, 3 and 4 stacked layers (to improve signal and check linearity).
2.3 Test Procedure
-
Mount 1–4 layers of separator in each channel; inject electrolyte and allow soak time under argon to ensure full wetting.
-
Apply the selected static preload (pressure) and equilibrate.
-
Record EIS (frequency sweep) and extract high-frequency intercepts to determine ionic resistance for each stack.
-
Repeat at several preset pressures (including the baseline near 0 MPa and up to the study’s maximum).
-
Fit resistance vs. number of layers to extract the per-layer ionic resistance R, then compute ionic conductivity σ with the standard relation below.
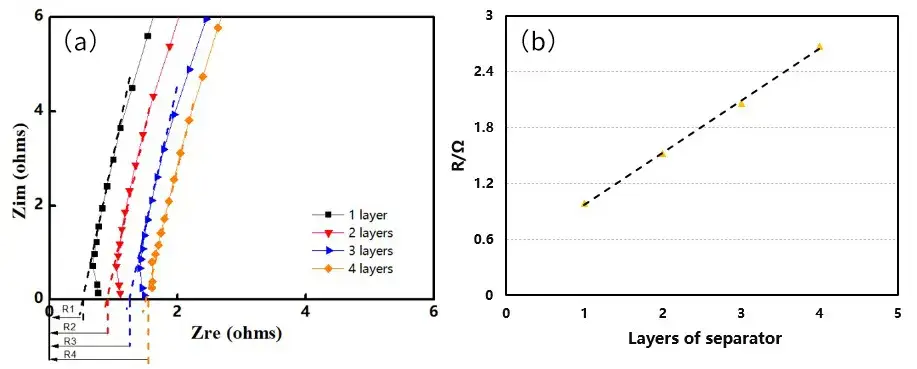
Figure 3. EIS impedance spectra of different diaphragm layers (a); R value fitting diagram (b)
2.4 Ionic conductivity calculation
σ=d /( R * S) (1)
where σ = ionic conductivity (S/cm), d = thickness (cm), R = ionic resistance (Ω), and S = electrode area (cm²).
In practice:
-
Measure R by linear fitting of impedance-derived intercepts for stacks of different layer counts (this reduces contact and fixture contributions).
-
Use the physical thickness ddd of a single separator layer (measured under the same pressure conditions if compression is significant).
-
Use the geometric electrode/separator contact area SSS used in the test fixture.
This approach provides robust ionic conductivity calculation and minimizes systematic error from contact resistances.
3. Results Analysis
3.1 EIS processing and extraction
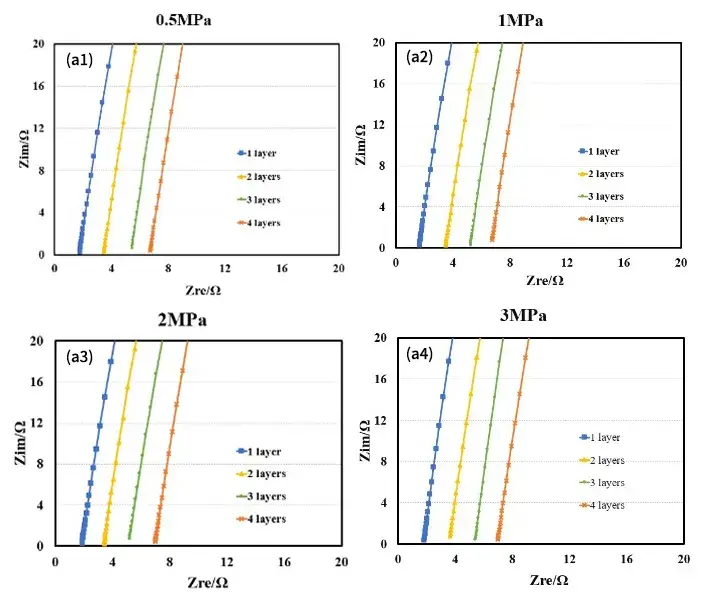
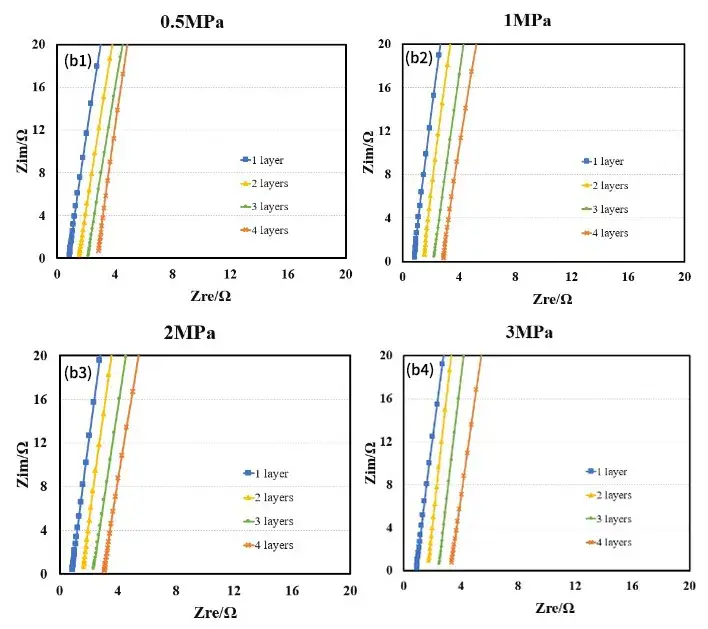
Figure 4. EIS spectra of diaphragms under different pressures: diaphragm A (a); diaphragm B (b)
Figure 4 shows the EIS spectra of separators A and B measured under different pressures. Using the obtained EIS as the baseline for linear fitting, the intersection values of the fitted line and the X-axis are recorded to obtain the impedance values R1, R2, R3, and R4 for 1 to 4 layers of the separator. Then, with the number of layers on the X-axis and R1, R2, R3, and R4 on the Y-axis, linear fitting is performed to obtain the ionic impedance R of the separators under different pressures, as shown in Tables 1 and 2. Substituting the R values into formula (1), the ionic conductivity of the separators under corresponding pressures is calculated, as illustrated in Figure 5. From the figure, it can be observed that as the pressure increases, the ionic conductivity of the separators decreases linearly, and the rate of decrease for separator B is significantly greater than that for separator A.
Table 1. Ionic resistance and ionic conductivity of separator A at different pressures
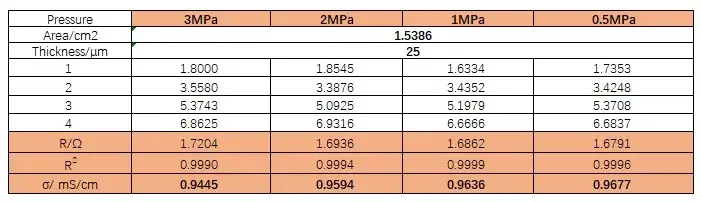
Table 2. Ionic resistance and ionic conductivity of separator B at different pressures 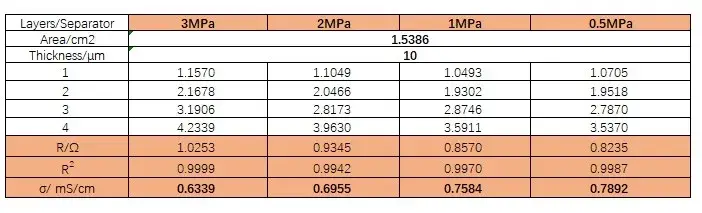
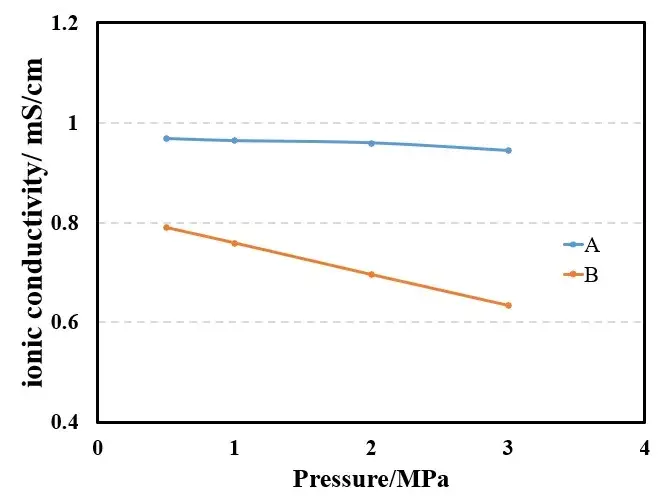
The porosity of the separator is a critical factor determining mass transport, as it ensures sufficient Li+ ion conductivity. The data from this experiment demonstrate that mechanical pressure alters the microstructure of the separator, hindering ion migration and thereby reducing the Li+ conductivity of the separator.
4. Discussion — mechanisms & practical implications
4.1 Mechanisms behind conductivity loss under compression
-
Porosity reduction: Compression reduces pore volume and connectivity, raising tortuosity and reducing continuous liquid pathways for ions.
-
Pore constriction / closure: Narrowing or closure of transport channels increases effective path length and resistance.
-
Contact changes: While using stacked layers and fitting reduces contact artifacts, local contact area changes between separator, current collector and fixture under load can slightly affect apparent R.
4.2 Why some separators are more sensitive
Separators with thinner pore walls, higher initial porosity, or less mechanically robust polymer matrices will compact more at low pressures and thus lose ionic conductivity faster. Reinforced or microporous separators with higher modulus resist pore collapse and maintain σ better under preload.
4.3 Cell and pack design implications
-
Module preload and spring design: Module clamping forces and spring pretension should be chosen to avoid compressive regimes where separator conductivity loss begins to significantly degrade performance.
-
Electrode-to-separator stack modeling: Battery models should include pressure-dependent σ to correctly predict rate capability and spatial state-of-charge gradients under pack compression.
-
Separator selection: For high-power or mechanically constrained designs, choose separators with lower pressure sensitivity (stiffer microstructure or reinforced architecture).
-
Formation & cycling protocols: Formation pressures and cell fixture loads should be controlled and reported since they can change the effective ionic conductivity baseline.
5. Best practices for measuring separator ionic conductivity under pressure
-
Use multi-layer stacking + linear fitting to remove fixture/contact artifacts and isolate per-layer R.
-
Measure actual thickness under load if possible — thickness change affects σ calculation directly.
-
Control atmosphere & wetting: run tests in an inert atmosphere and allow full electrolyte equilibration before EIS.
-
Report test details: pressure, soak time, electrolyte composition, temperature, sample area and layer count — these strongly affect reproducibility.
-
Map sensitivity: characterize σ(p) over the expected pressure range for your cell/pack to define safe operating preload windows.
6. Conclusion
This study demonstrates that mechanical pressure substantially affects separator ionic conductivity, with significant variability between materials. Using the EIC2400M system, we accurately measured conductivity changes under pressure, accurate ionic conductivity calculation based on multi-layer EIS fitting provides a practical way to quantify this effect and inform separator choice, module preload specification, and electrochemical modeling for high-power cells.
These insights can guide the development of pressure-resilient separators and inform battery assembly processes, ultimately contributing to improved performance and longevity of lithium-ion batteries.
7. References
[1] Cui Jin, Shi Chuan, Zhao Jinbao. Research progress on the effect of mechanical pressure on the performance of lithium batteries[J]. Journal of Chemical Industry, 2021, 72(7): 3511-3523.
[2] Gioia G, Wang Y, Cuitiño A M. The energetics of heterogeneous deformation in open-cell solid foams[J]. Proceedings of the Royal Society of London Series A: Mathematical, Physical and Engineering Sciences, 2001, 457(2009): 1079-1096.
[3] Sarkar A, Shrotriya P, Chandra A. Modeling of separator failure in lithium-ion pouch cells under compression[J]. Journal of Power Sources, 2019, 435: 226756.
[4] Peabody C, Arnold C B. The role of mechanically induced separator creep in lithium-ion battery capacity fade[J]. Journal of Power Sources, 2011, 196(19): 8147-8153.
8. Lithium Battery Testing Solutions Recommend:IEST Instrument
IEST is a high-tech enterprise focusing on R&D and production of lithium ion battery tester. IEST is a professional manufacturer that integrating laboratory instrument R&D and production, method development, instrument sales and technical services. Committed to providing leading testing solutions and services for the global new energy field, IEST company serves key clients like CATL, BYD, Huawei, Svolt, GM, Leyden, Sintef, Factorial, Cabot, Cuberg.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.





