-
iestinstrument
Redox-Active Stabilizer–Enhanced Structural and Thermal Stability of Ni-Rich Cathodes via an Economical Blending Strategy
1. Article Information
First Author: Jinzhong Liu
Corresponding Authors: Jinyang Dong*, Meng Wang*, Lai Chen*
Affiliations: Beijing Institute of Technology, Chongqing Innovation Center of Beijing Institute of Technology, China North Vehicle Research Institute, IEST Instrument
Instruments: BER Series Battery Electrode Sheet Resistance Tester & EWS Series Electrolyte Wettability Testing System (IEST Instrument)
2. Research Background
Lithium-ion batteries (LIBs) are widely utilized, and Ni-rich cathode materials have garnered significant attention due to their high energy density. However, challenges such as structural degradation (e.g., irreversible phase transitions, interfacial cracking) and thermal instability (e.g., parasitic reactions, oxygen release during thermal runaway) persist. Existing modification strategies are often complex and costly, driving the demand for more efficient and cost-effective solutions. This has spurred research into blending high-capacity/low-stability cathodes with high-stability/low-reactivity cathodes. Currently, there is a lack of scientific guidance and well-defined design criteria, as the mechanisms by which blended cathodes achieve enhanced structural and thermal stability remain insufficiently studied. Without an in-depth understanding of how different cathode materials interact during blending, it is challenging to optimize their performance and longevity.
3. Work Summary
Researchers from Beijing Institute of Technology (Prof. Lai Chen, Dr. Jinyang Dong) and Chongqing Innovation Center (Dr. Meng Wang) proposed an innovative blending strategy. By incorporating 10 wt% LiFePO₄ (LFP) as a “redox-active stabilizer” into layered NCM811, the following benefits were achieved:
- Interfacial Suppression: LFP forms a coating-like layer that mitigates interfacial side reactions.
- Buffering Effect: LFP regulates lithium-ion intercalation kinetics, reducing mechanical strain.
- Structural Reinforcement: The strong P-O covalent bonds in LFP enhance structural and thermal stability while preserving energy density.
This work, titled “Redox-active stabilizer-enhanced structural and thermal stability of Ni-rich cathodes via an economical blending strategy“, was published in eTransportation.
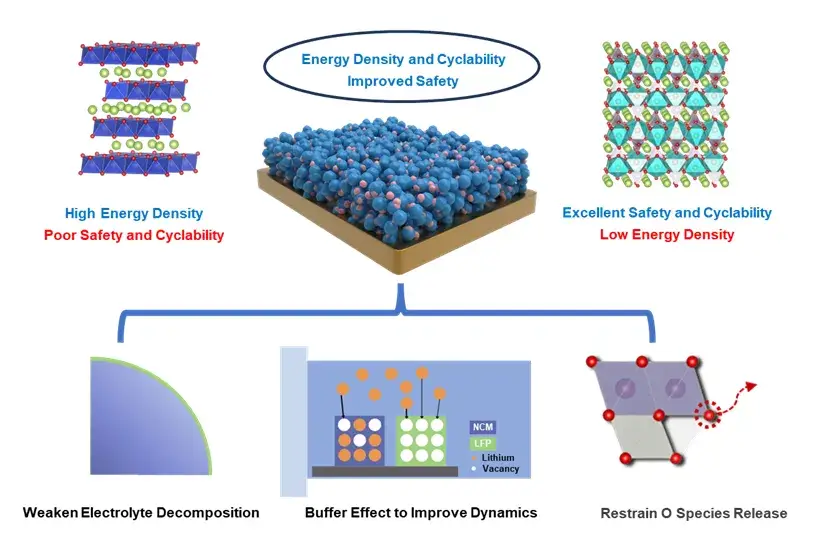
Figure 1. Schematic of LFP modification mechanism.
3. Key Findings
3.1 Morphological and Structural Characterization
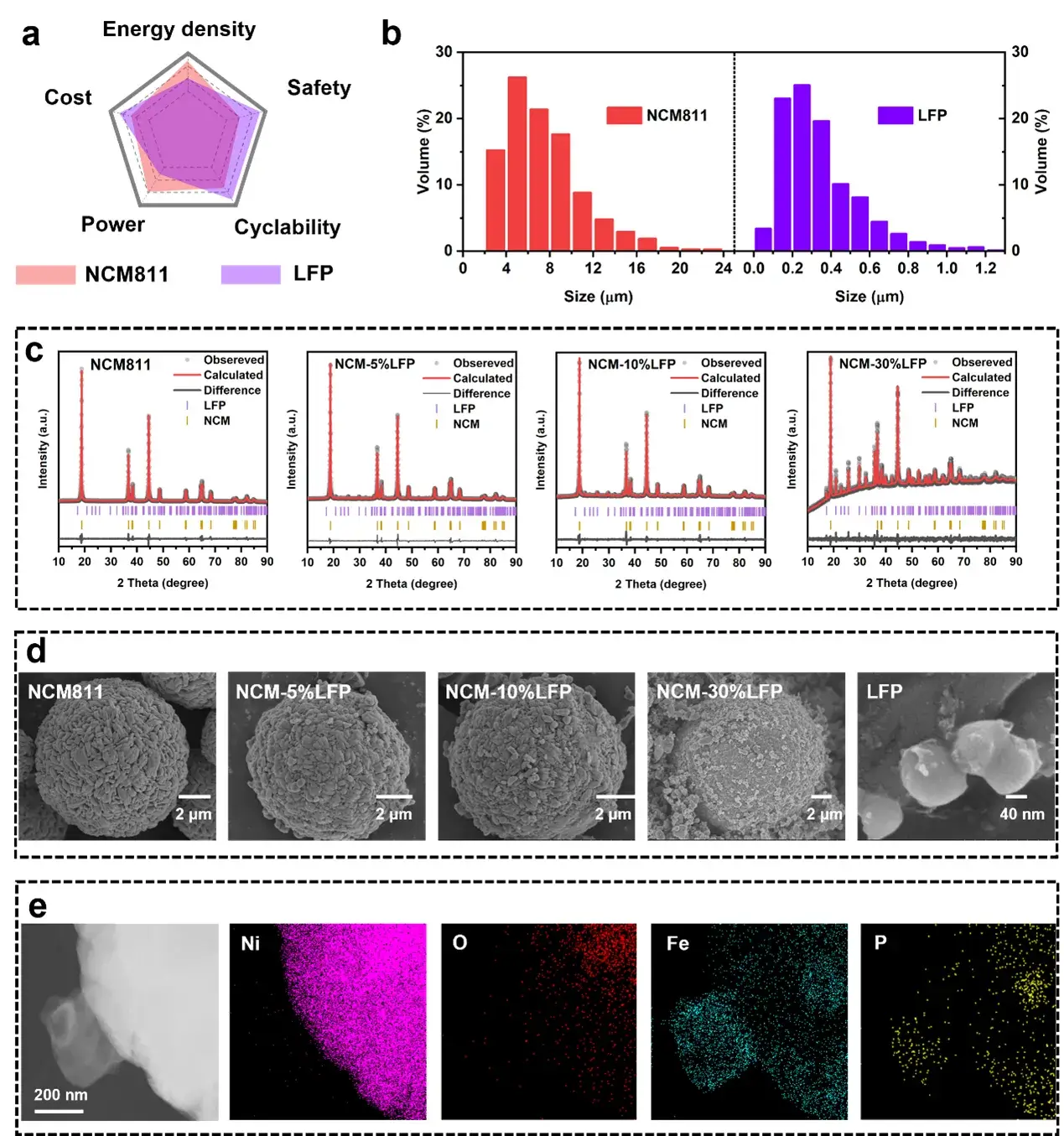
Figure 2. Morphological and structural characterization.
SEM (Scanning Electron Microscopy) and TEM (Transmission Electron Microscopy) analyses confirm the presence of a uniform coating-like layer on the material surface. At lower LFP contents (NCM-5%LFP and NCM-10%LFP), the LFP primarily exists as a quasi-coating layer, while at higher LFP content (NCM-30%LFP), partial LFP agglomeration is observed. XRD (X-ray Diffraction) analysis further demonstrates that mechanical ball milling does not compromise the lattice structures of NCM811 or LFP.
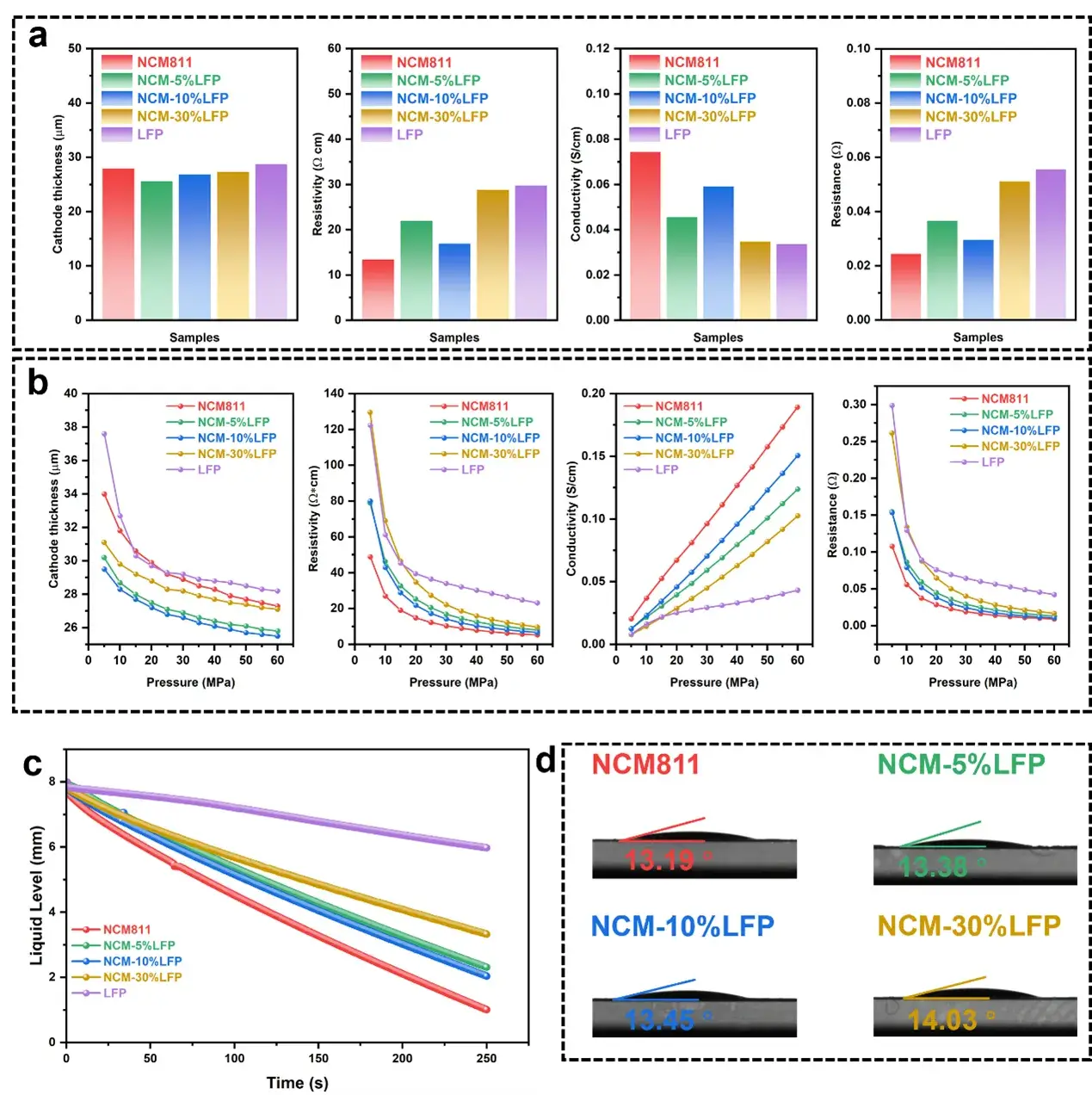
Figure 3. Evaluation of electrode thickness, resistance, resistivity and conductivity.
The BER series electrode resistance Tester(IEST Instrument) was utilized to evaluate the thickness, resistance, resistivity, and conductivity of the electrode sheets. Among the composite materials, the NCM-10%LFP exhibited the lowest resistivity. Additionally, the EWS1100 series electrolyte wettability testing system (IEST Instrument) was employed to assess the electrolyte wettability of the electrodes.
3.2 Rate-Dependent Failure Behavior
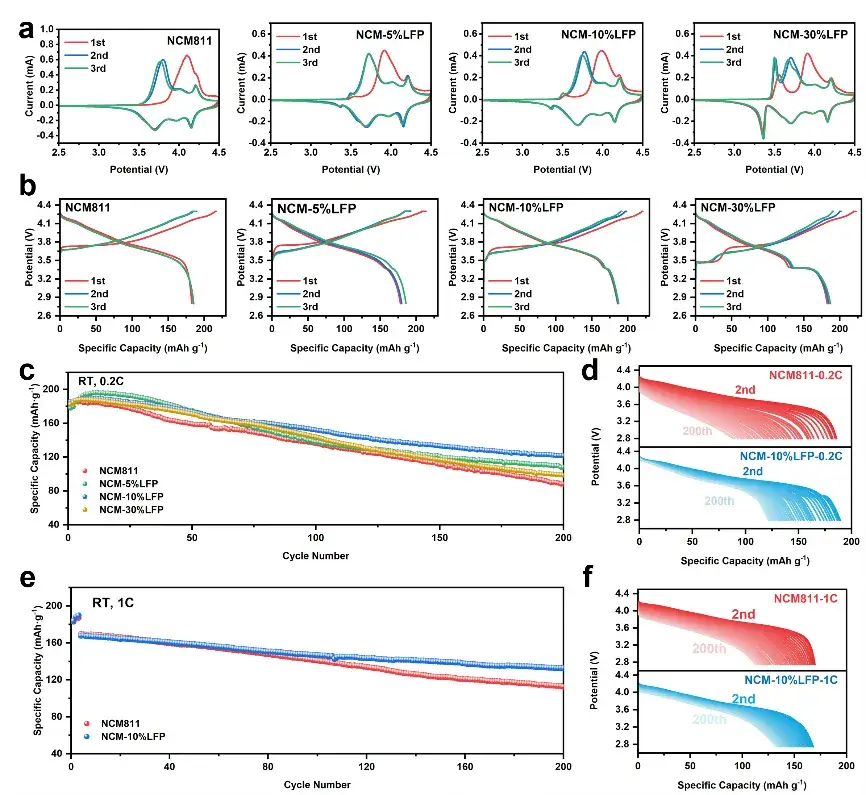
Figure 4. Surface composition and structural evolution during initial charge/discharge.
Half-cells were assembled to test the electrochemical performance, and the Cyclic voltammetry (CV) curves showed that LFP enhanced the electrochemical kinetics; NCM-10%LFP demonstrated a slight increase in initial capacity and significantly improved cycling stability.
3.3 Failure Mechanism Analysis
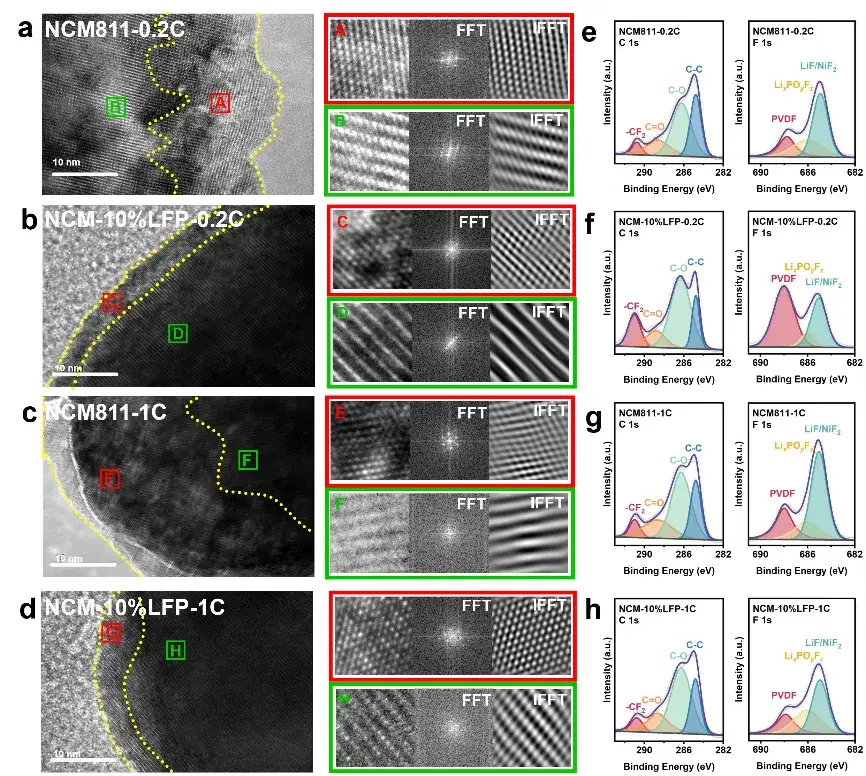
Figure 5. Post-cycling SEM, TEM, and XPS characterization.
SEM and TEM characterization revealed that LFP effectively mitigates structural degradation (e.g., reduced microcracking) in NCM811. Meanwhile, XPS (X-ray Photoelectron Spectroscopy) and DSC (Differential Scanning Calorimetry) analyses demonstrated that LFP suppresses parasitic reactions at the electrode-electrolyte interface.
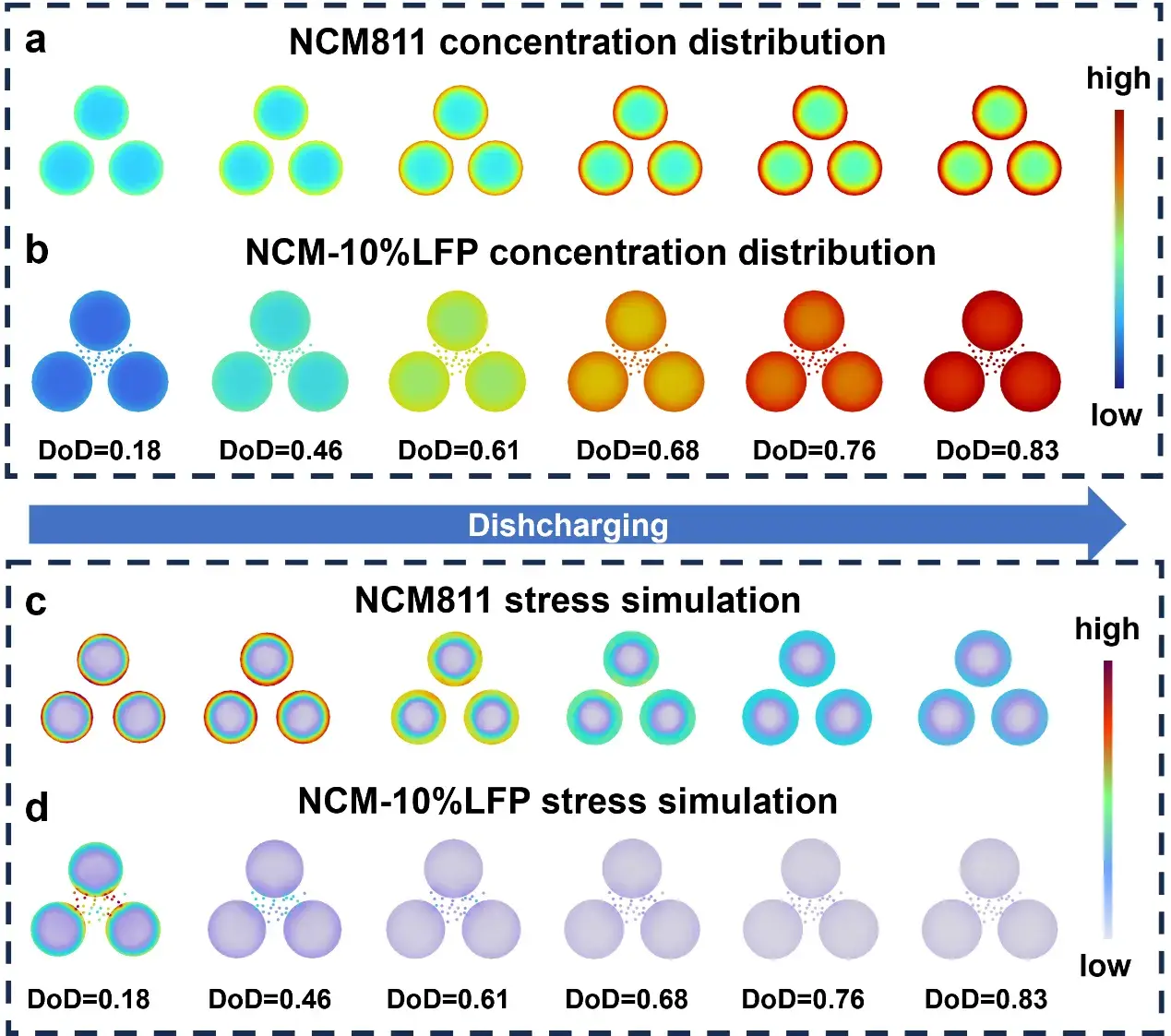
Figure 6. Simulation results
The study revealed that LFP functions as a buffering agent in the composite cathode material, effectively improving Li⁺ diffusion kinetics. Multi-physics simulations visually demonstrated the impact of LFP modification on ion transport and mechanical behavior, thereby elucidating the mechanism behind its enhanced performance.
4. Conclusions
The blending strategy proposed in this study is simple and cost-effective. The prepared LFP-NCM811 composite cathode material exhibits enhanced structural and thermal stability, along with improved cycling durability. Specifically, the NCM-10%LFP demonstrates high capacity retention after 200 cycles at both 0.2C and 1C rates. This work establishes a novel framework for developing commercial cathode materials that integrate high energy density, enhanced safety, and low cost.
5. Publication Details
Jinzhong Liu, Jinyang Dong, Meng Wang, Na Liu, Haoyu Wang, Kang Yan, Hongyun Zhang, Xi Wang, Rui Tang, Yun Lu, Qiongqiong Qi, Yuefeng Su, Feng Wu, Lai Chen. Redox-active stabilizer-enhanced structural and thermal stability of Ni-rich cathodes via an economical blending strategy [J]. eTransportation, 2025, 24: 100428.
6. Related Testing Instruments Recommendation
IEST Lithium Battery Electrode Sheet Resistance Tester(BER Series)
Introduction: Combined with high-precision pressure control, thickness and resistance testing systems, the dual-plane controllable pressure disc electrode resistance method is used to test the overall penetration internal resistance of the electrode, including coating resistance, interface resistance between coating and current collector, and current collector resistance. The sum can be used for electrode sheets formulation development and process stability monitoring.
Features:
- Create a new method for testing electrode resistance to evaluate the uniformity of the electrode conductive network;
- Fully automatic testing software, parameters can be set freely and can be started with one click;
- Real-time monitoring and output of pressure, pressure, ambient temperature, ambient humidity, thickness, resistance, resistivity, conductivity, compaction density and other parameter curves, and automatically save test data.
- Equipped with standard thickness blocks and resistance blocks calibrated by a third-party metrology institute.
IEST Electrolyte Wetting Testing System(EWS/ETS/CHT)
Introduction: Combining the visual acquisition system and the capillary action of the electrolyte to evaluate the wetting differences of different electrodes, electrolytes, diaphragm formulations and processes.
Features:
- Based on the principle of capillary diffusion of electrolyte in the electrodes and separators, quantitatively evaluate the difference in electrolyte infiltration;
- Equipped with high-precision mechanical control and visual acquisition system, the test is stable and efficient;
- Suitable for evaluation of infiltration differences of different electrodes, electrolytes, separator formulas and processes;
- Characterize the infiltration rate of electrolyte in the sample in real time.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.