Description

1. Significance of Ion Conductivity Testing
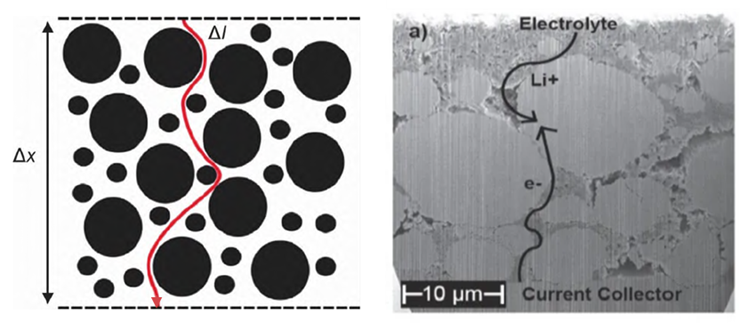
Electrode Tortuosity and Kinetic Performance:
- Tortuosity represents the degree of bending in the transport pathways within a porous electrode. It is a critical parameter, alongside porosity, governing transport properties. It characterizes the percolation capability of the electrolyte and the ion migration rate, directly impacting the battery’s capacity utilization and rate capability.
- Measuring the pore tortuosity of an electrode enables performance prediction. This facilitates the rapid correlation of electrode structure with expected performance, accelerating electrode design and process development.
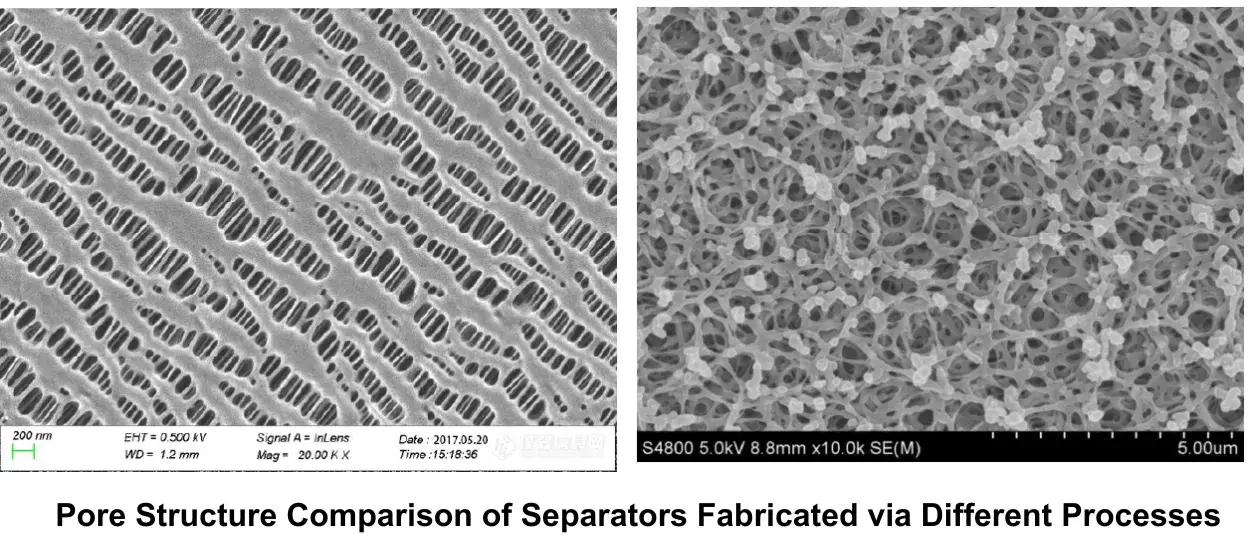
Separator Ionic Conductivity:
- In recent years, separator coating applications have expanded significantly. Coating processes enhance lithium-ion battery separator properties such as puncture resistance, thermal stability, and electrolyte wettability. While improving safety performance, it is equally crucial to ensure stable electrochemical performance. Therefore, ionic conductivity testing is particularly important for comparing and characterizing separator performance.
2. Testing & Calculation Methods
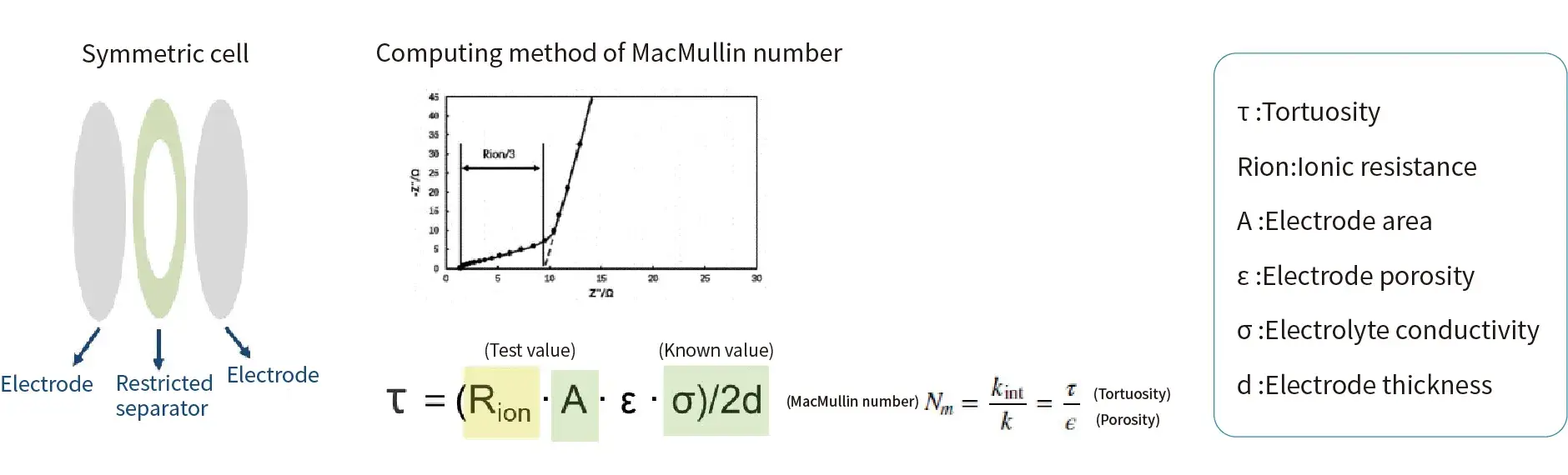
2.1 Electrode Testing
-
Assemble a symmetrical cell and perform Electrochemical Impedance Spectroscopy (EIS) testing.
-
As shown in the figure below, perform linear fitting on the high-frequency region and the low-frequency region of the EIS spectrum (Nyquist plot). Three times the difference between the points where the respective fitted curves intersect the real impedance axis (Z’/X-axis) gives the ionic resistance Rion of the electrode coating.
-
Calculate the MacMullin number using the formula to indirectly characterize the electrode tortuosity.
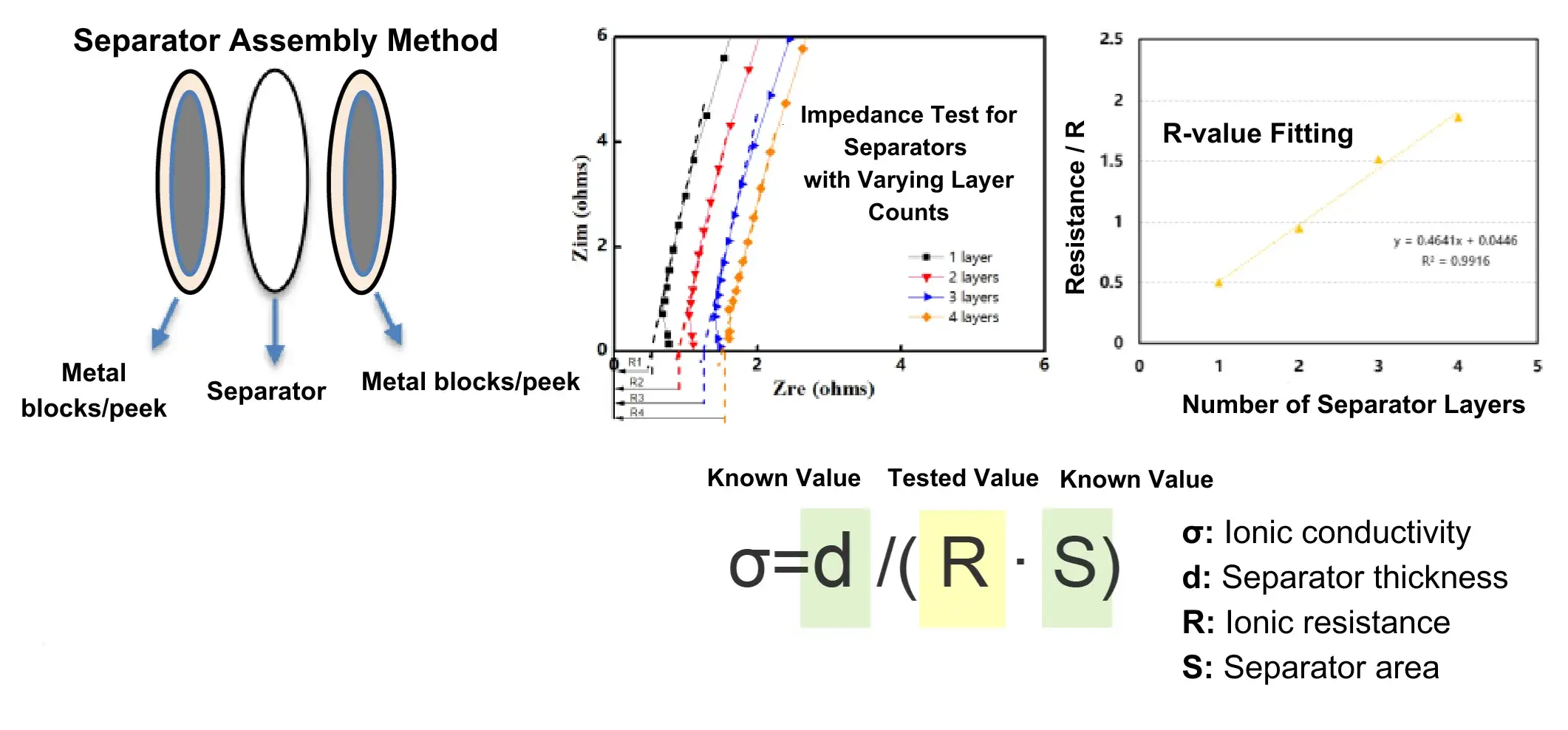
2.2 Separator Testing
- Measure the impedance of separators with 1 to 4 layers, obtaining values R₁, R₂, R₃, R₄.
- Plot separator resistance (R) versus number of separator layers on the vertical and horizontal axes, respectively.
-
Determine the slope of the curve and its linearity of fit.
-
The linear regression coefficient (R²) must be ≥0.99.
-
- Calculate the ionic conductivity (σ) using the formula: σ = d / (R × S)
3. IEST Creative Solutions
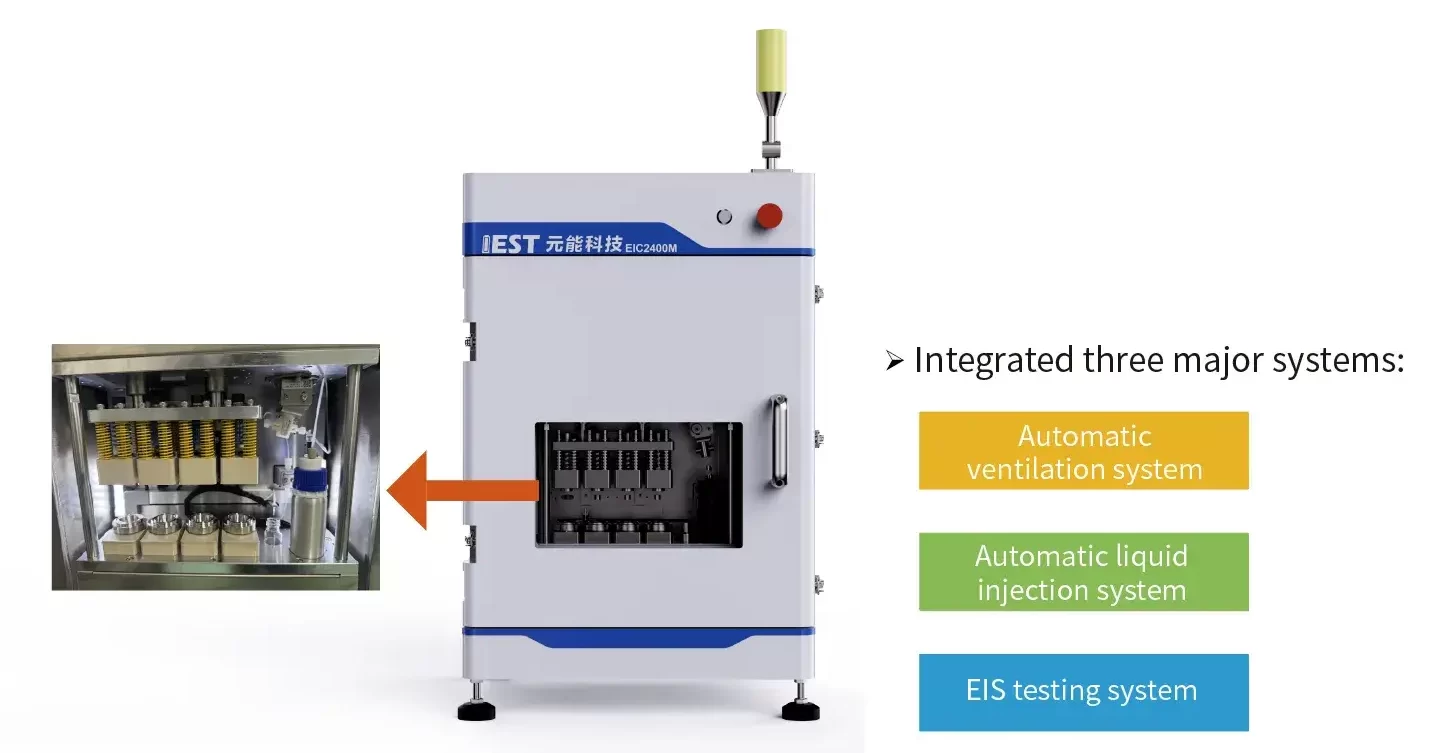
3.1 Creative Solution one
- Calculate electrode tortuosity measurement from electrochemical impedance spectroscopy (EIS) of symmetric cells.
- Streamlined cell assembly, automated testing and analysis, simplified operation workflow, enhanced testing throughput.
- Four-channel synchronous measurement
3.2. Creative Solution Two
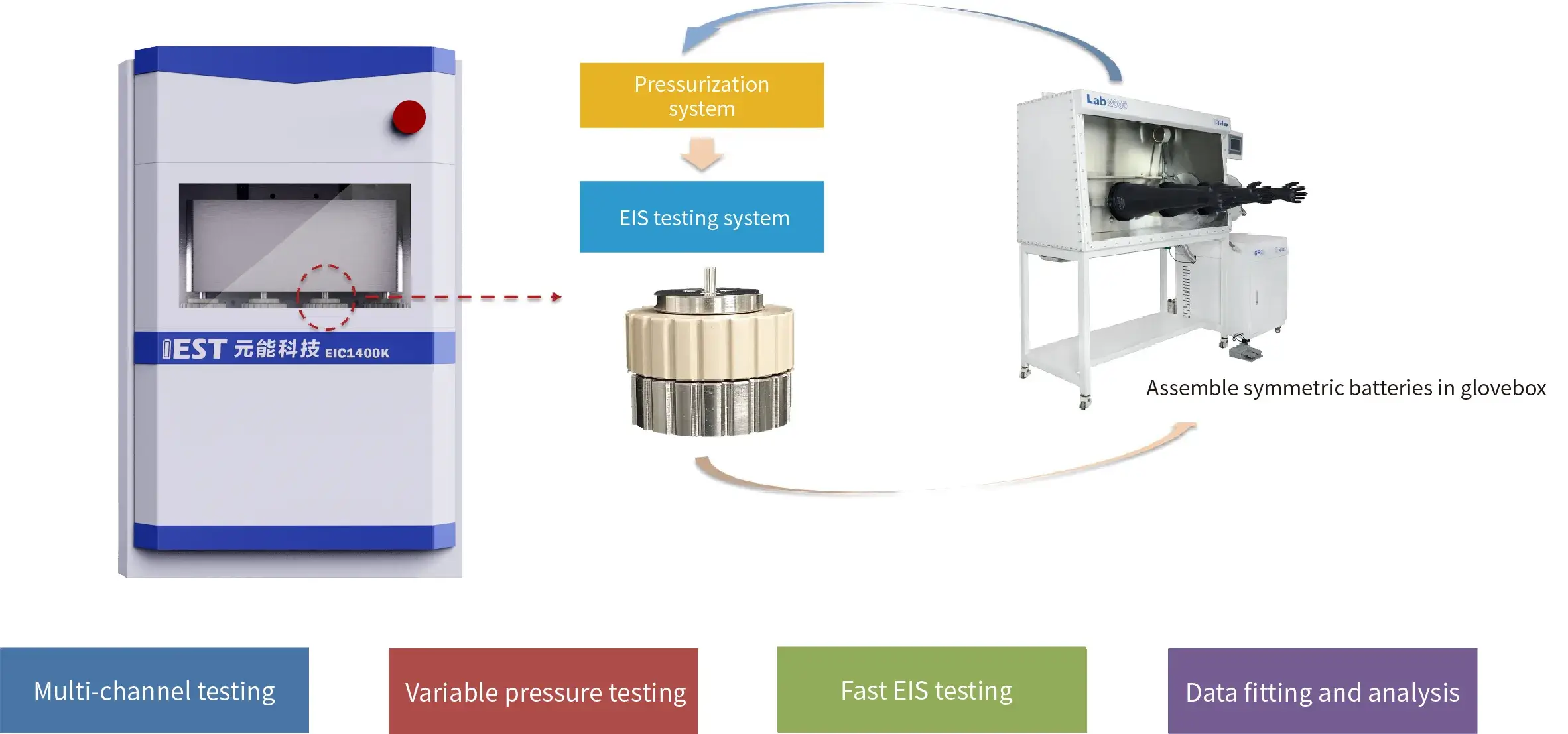
Applications
Case 1. Different compaction density of cathode electrodes(NCM)
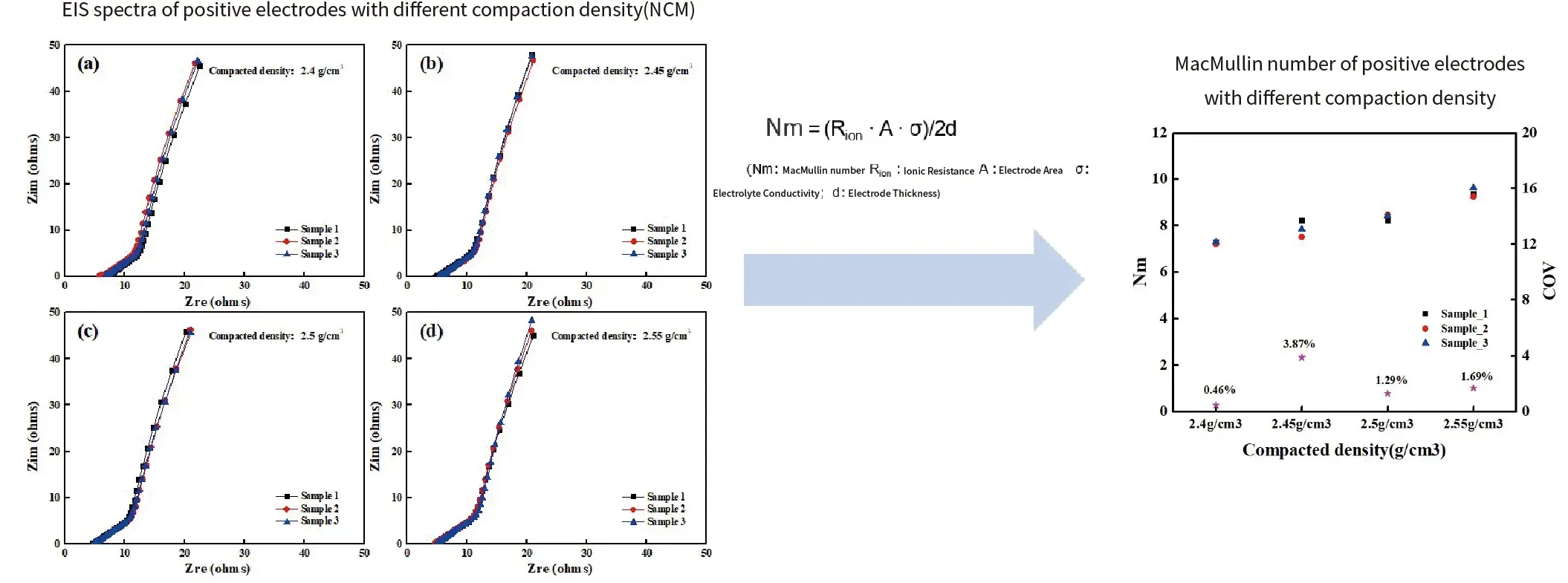
Summary:
- The consistency of ElS testing for symmetric battery of electrodes is generally good.
- Within a certain range of compaction density, as the compaction increases, the ionic resistance/MacMullin number also increases.
Case 2. Different compaction density of anode electrodes(Gr)
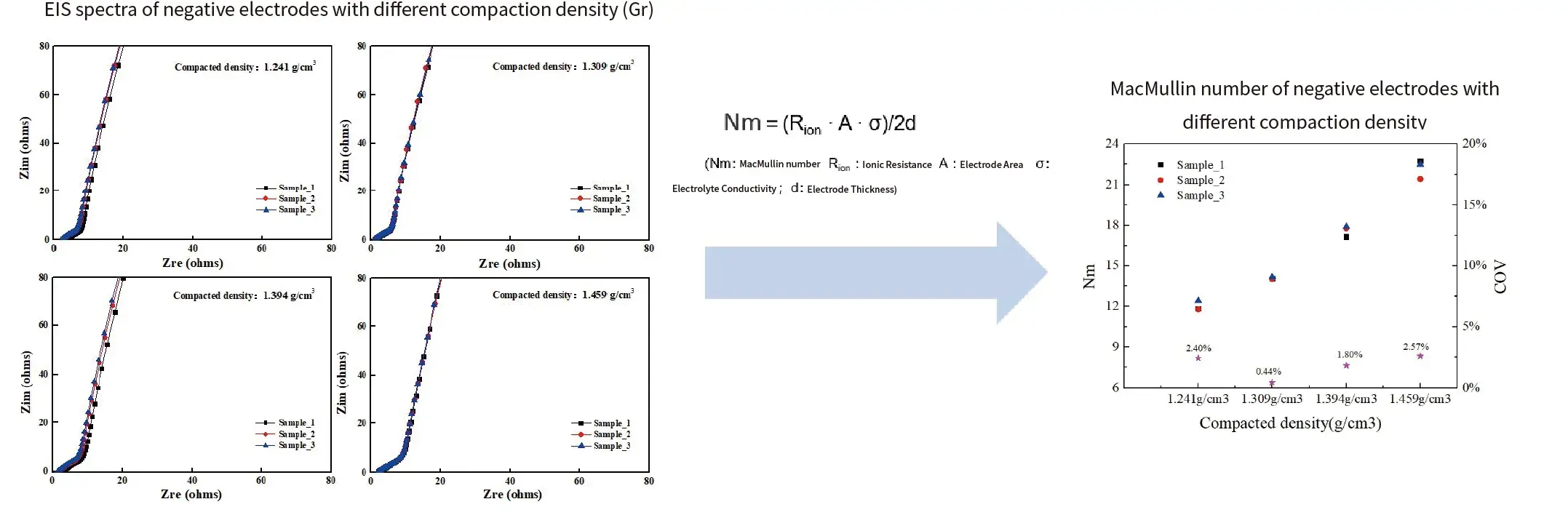
Summary:
- The consistency of ElS testing for symmetric battery of electrodes is generally good.
- Within a certain range of compaction density, as the compaction increases, the ionic resistance/MacMullin number also increases.
Case 3. The correlation between electrode tortuosity and electrochemical performance(Gr anode electrodes of different thicknesses)
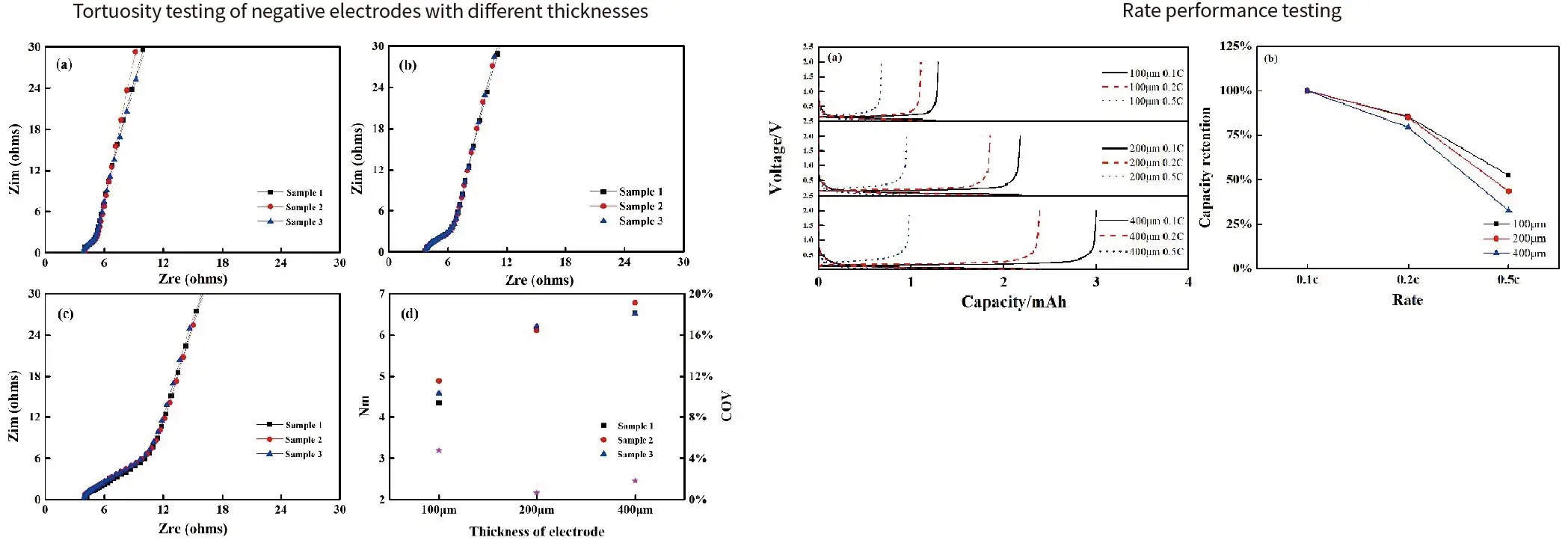
Summary:
- As the thickness of the electrode increases, its tortuosity also increases, However, the rate performance of the battery decreases with increasing thickness.
- This indicates that the rate performance of the battery decreases with increasing tortuosity. There is a certain correlation between electrode tortuosity and rate performance of battery.
Case 4. Ionic Conductivity Comparison of Separators with Four Different Coatings
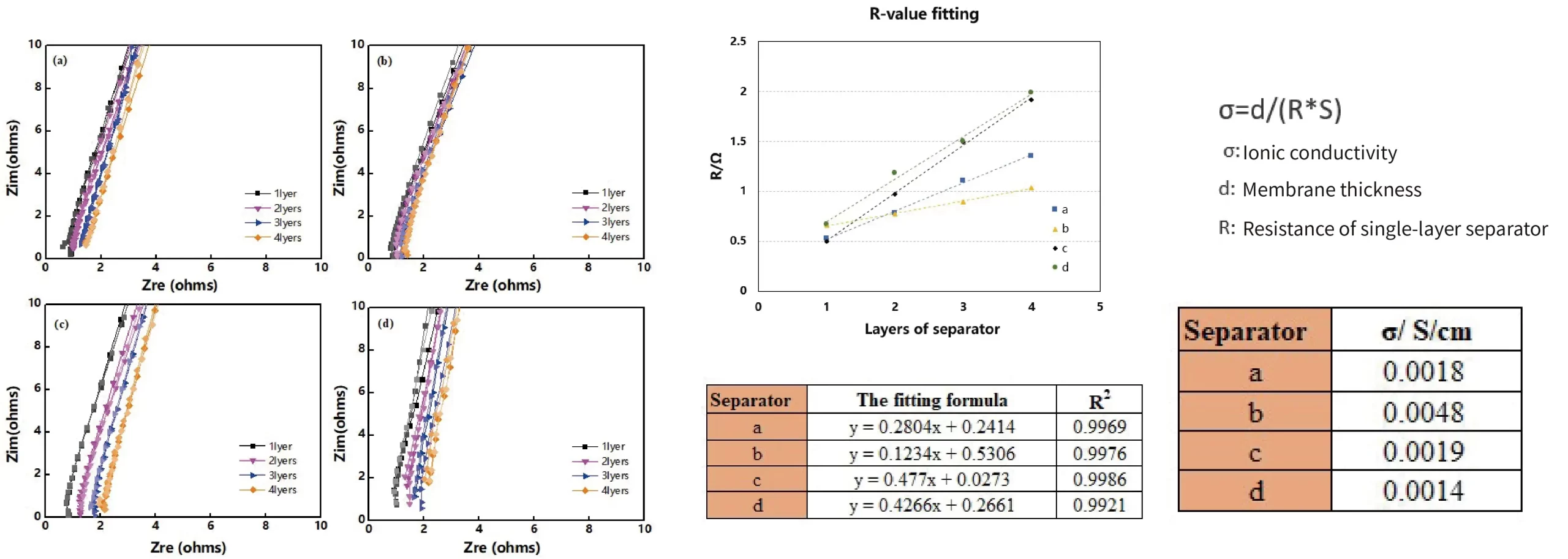
Summary:
- Test the EIS of 1-4 layers of separators to obtain R1, R2, R3, R4.
- Plot a curve with the number of separator layers as the x-axis and separator resistance as the y-axis. Calculate the slope and linear fitting degree of the curve, with the linear fitting degree ≥0.99.
- Calculate the separator ionic conductivity according to the formula.
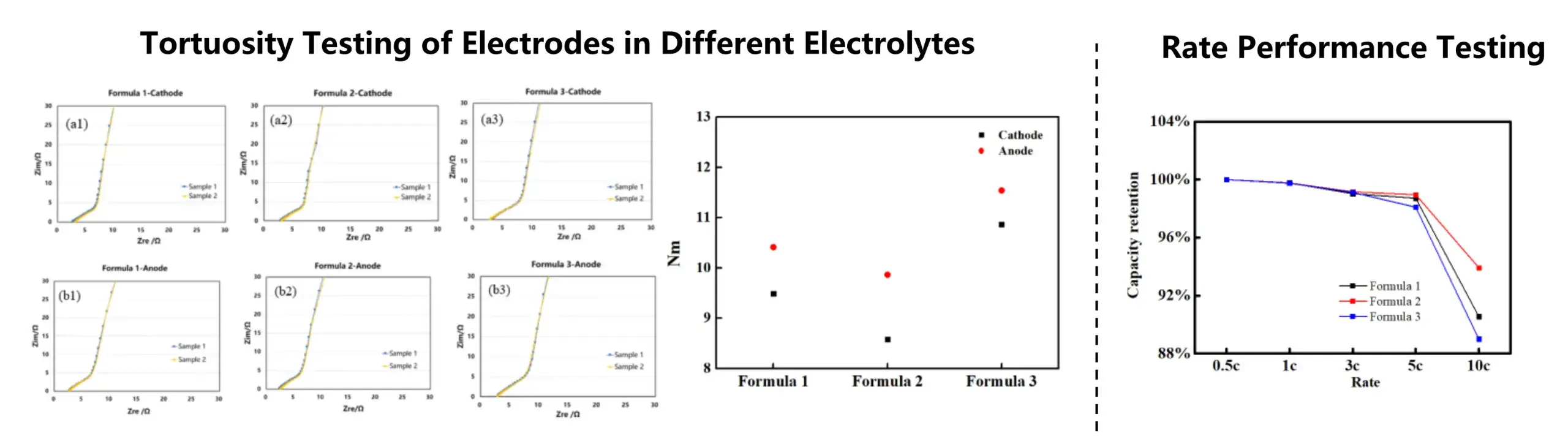
Case 5. Correlation Between Electrode Tortuosity and Electrochemical Performance in Different Electrolytes
Summary:
-
The MacMullin number of both the cathode and anode electrodes in the electrolyte follows the order: Formulation 2 < Formulation 1 < Formulation 3.
-
At a 10C rate, the capacity retention of Formulation 1 is 90.55%, Formulation 2 is 93.92%, and Formulation 3 is 89%.
-
Combined with the MacMullin number data, it can be concluded that the ease of lithium-ion migration within the coating is influenced by the electrolyte formulation. A higher MacMullin number corresponds to poorer battery rate performance, indicating consistency between the electrochemical test results and tortuosity measurements.
Case 6. Tortuosity & Wettability of LFP Cathodes with Different Compaction Densities
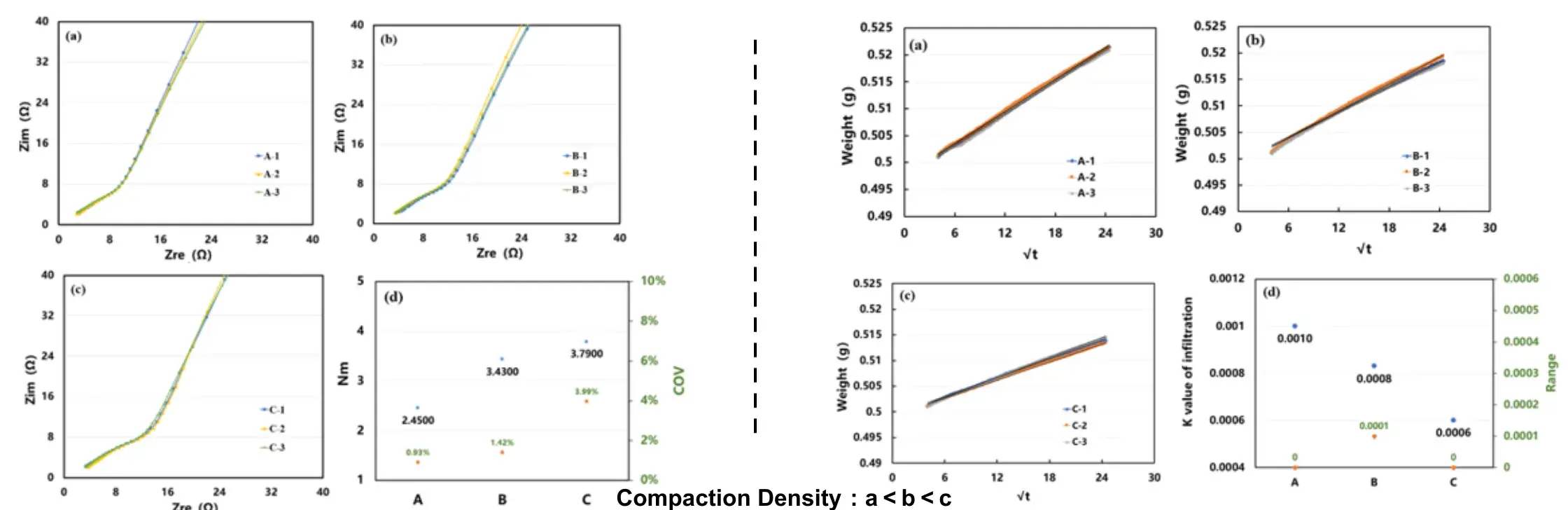
Summary:
- Electrodes with poorer wetting performance exhibit higher tortuosity.
- As the compaction density increases, the electrolyte absorption performance deteriorates, making electrolyte infiltration more difficult. This hinders lithium-ion migration, increases ion transport resistance, and ultimately leads to higher electrode tortuosity.
Video