-
iestinstrument
How to Rapidly Evaluate Low Temperature Electrolyte Performance for Lithium-ion Batteries
1. Abstract
This article presents a faster, more efficient method for screening low temperature electrolyte candidates. By measuring the separator ionic conductivity with different electrolytes across a temperature gradient, the entire evaluation process can be completed in approximately 30 minutes, dramatically accelerating R&D iteration. The method reproduces the electrolyte’s behavior inside a porous separator, making it relevant to practical cell performance.
2. Introduction: The Challenge of Battery Performance in the Cold
Lithium-ion batteries power our portable electronics, electric vehicles, and energy storage systems. However, their performance degrades significantly in cold environments. Key issues include reduced ionic conductivity, accelerated lithium dendrite growth, and increased parasitic reactions at electrode interfaces. These limitations restrict the use of LIBs in applications exposed to extreme temperatures.
The electrolyte—a mixture of lithium salts, solvents, and additives—is central to solving this challenge. Its composition dictates lithium-ion transport kinetics and the stability of the electrode-electrolyte interface. Therefore, optimizing the electrolyte formulation is a primary strategy for enhancing low-temperature performance. This typically involves employing low-viscosity solvents, high-conductivity lithium salts, and functional additives.
Traditional evaluation methods, such as measuring bulk electrolyte conductivity and viscosity at low temperatures followed by full-cell cycling tests, are time-consuming, often taking 3-7 days. This extended timeline hinders rapid research and development cycles.
3. Methodology: A High-Throughput Testing Approach
3.1 Test Equipment
The core of this rapid assessment is the EIC2400M-T multi-channel ionic conductivity test system (IEST). This instrument features four independent test channels, operates within a high-purity argon atmosphere, and performs automated Electrochemical Impedance Spectroscopy (EIS) measurements. Its operational range spans -20°C to 80°C.
Figure 1. IEST Electrode Tortuosity Tester & Separator Ion Conductivity Tester(EIC Series)
3.2 Test Samples & Procedure
We compared a baseline electrolyte (Electrolyte A) against a specialized low temperature electrolyte (Electrolyte B) using a standard commercial separator.
-
Separator stacks of 1, 2, 3, and 4 layers were prepared and placed into the four channels.
-
Close the test chamber and evacuate, then refill with high-purity argon to remove moisture.
-
Dose each channel with a controlled volume of electrolyte and allow a fixed wetting/soak time.
-
Acquire EIS for each channel and fit the spectra to extract the series resistance intercept Rs(n) for n separator layers (see Fig. 2(a)).
-
Plot Rs(n) versus layer number n and perform a linear fit; the slope R of that fit corresponds to the ionic resistance of one separator layer (see Fig. 2(b)).
-
The separator ionic conductivity (σ) is calculated using the formula: σ = d / (R * S), where σ is ionic conductivity, d is separator thickness, R is the single-layer ionic resistance from the fit, and S is the active area.

Figure 2. EIS impedance spectra of different numbers of membrane layers (a); R-value fitting plot (b)
4. Results & Analysis: Quantifying Low-Temperature Improvement
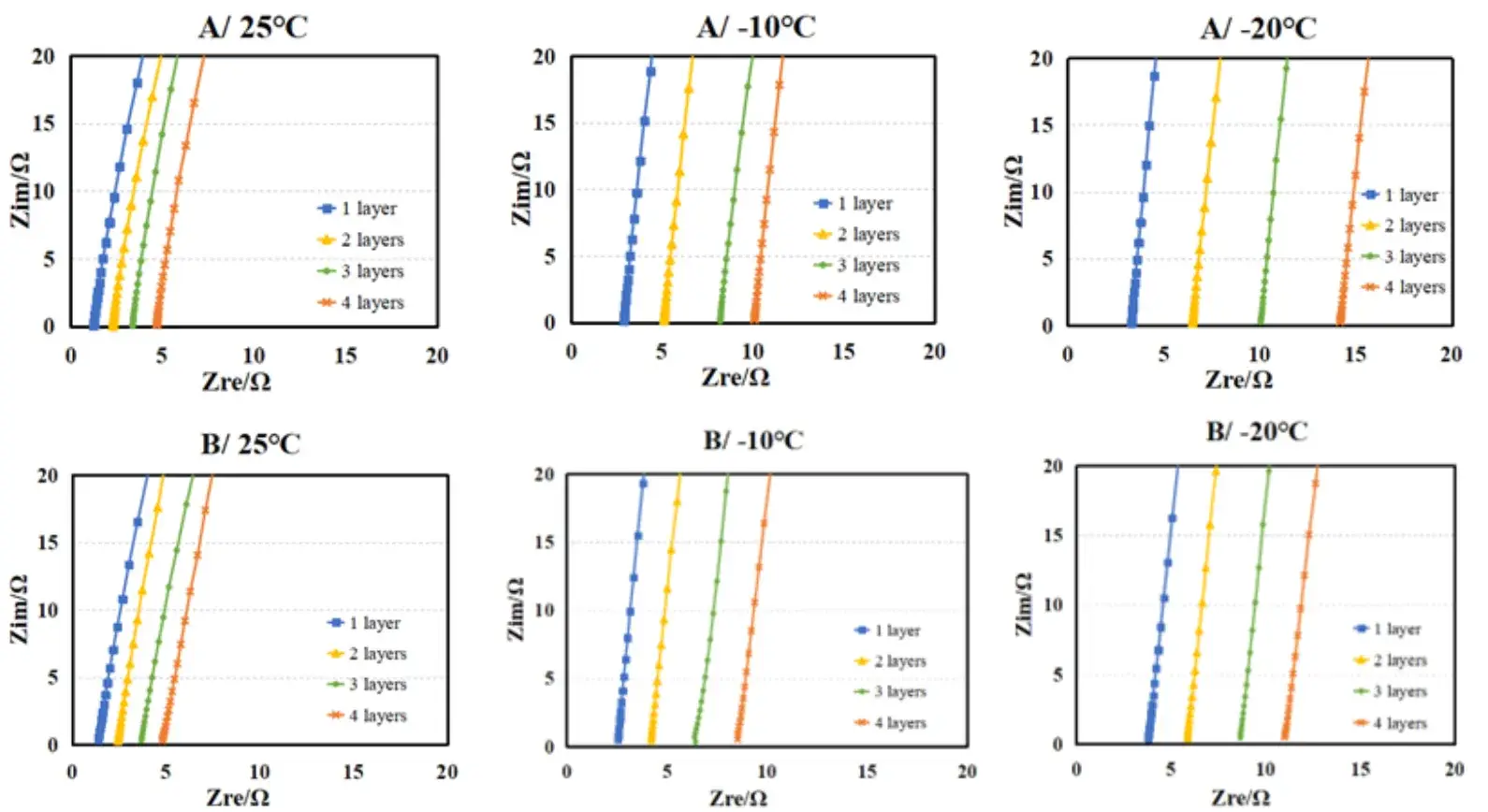
Figure 3. EIS spectra of electrolytes A and B at different temperatures.
EIS spectra were recorded for both electrolytes at multiple temperatures; representative spectra are shown in Figure 3. After fitting, we computed membrane ionic conductivity at each temperature for Electrolyte A and Electrolyte B (tabulated in Table 1 and plotted in Figure 4). Key quantitative observations are:
-
As expected, ionic conductivity decreased for both electrolytes as temperature dropped.
-
However, the rate of decline differed significantly. For Electrolyte A, the conductivity at -20°C was only 31.58% of its value at 25°C.
-
In contrast, Electrolyte B retained 47.14% of its room-temperature conductivity at -20°C.
-
This marked improvement demonstrates that Electrolyte B’s formulation—presumably containing optimized solvents and additives—effectively enhances low-temperature transport kinetics. The additives likely work by reducing electrolyte viscosity, optimizing the lithium-ion solvation structure for lower migration energy barriers, and improving separator wettability at cold temperatures.
Table 1. Separator ionic conductivity of electrolytes A and B at different temperatures
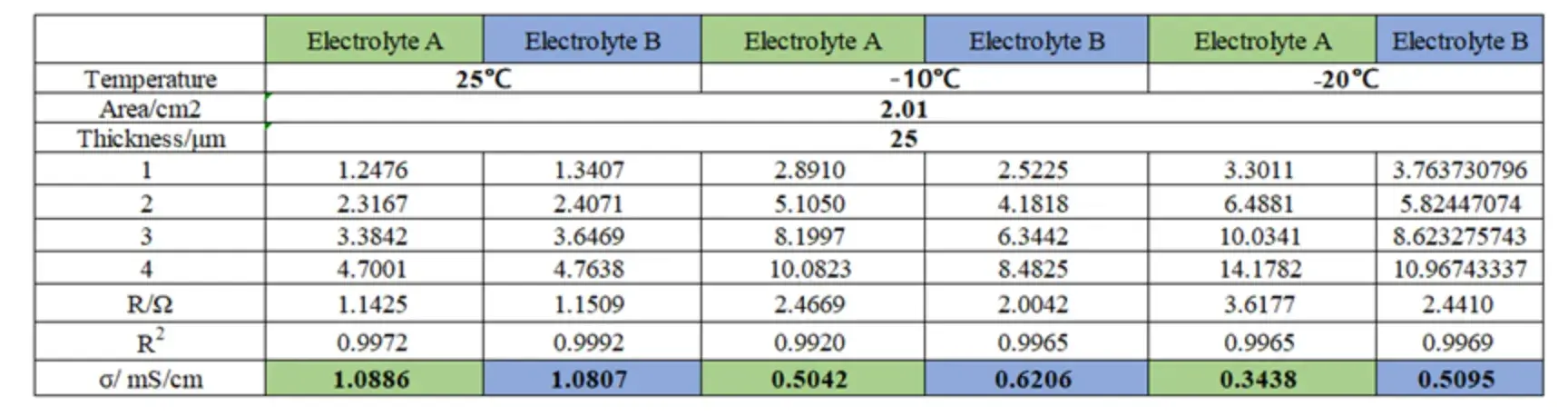
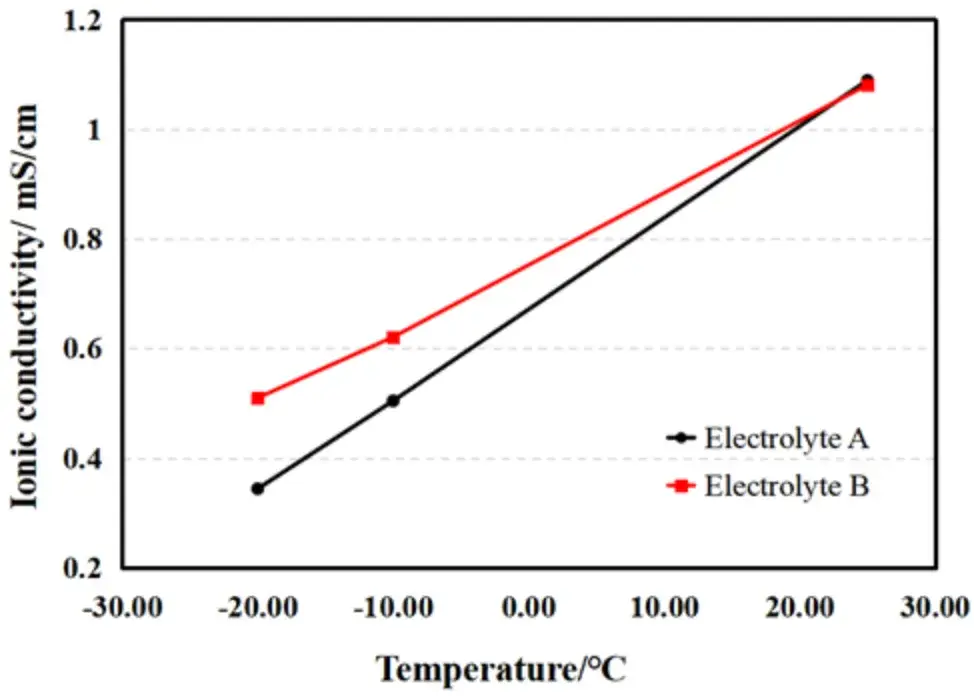
Figure 4. Changes in separator ionic conductivity of electrolytes A and B at different temperatures.
5. Discussion: interpretation and practical implications
The separator -EIS screening method measures electrolyte ionic transport within the real porous geometry of a separator rather than in a bulk conductivity cell; therefore, it captures two performance-critical factors simultaneously:
-
Bulk ionic mobility (solution conductivity): improved by low-viscosity solvents and high lithium salt dissociation.
-
Interfacial/wetting behavior: improved electrolyte wetting reduces tortuous path effects and contact resistance inside separator pores.
Electrolyte B’s superior retention of ionic conductivity at −20 °C implies that it will better support low-temperature charge/discharge processes in full cells, all else equal. However, ionic conductivity is one of multiple metrics that determine cell low-temperature performance: interphase stability (SEI/CEI), charge transfer resistance, and electrode kinetics must also be assessed in follow-up cell-level tests. This separator screening method should therefore be used as a rapid triage tool to prioritize candidate formulations for more extensive cell cycling tests.
6. Conclusion
This case study demonstrates a rapid and effective method for screening low temperature electrolyte formulations. By measuring separator ionic conductivity across a temperature range, researchers can clearly differentiate the low-temperature performance of different electrolytes in a fraction of the time required for full-cell testing.
The method’s strength lies in its practical relevance: it evaluates ionic transport within the porous separator matrix, closely simulating real battery conditions. This approach provides a reliable, high-throughput tool for the efficient development and selection of advanced electrolytes designed for superior cold-weather battery operation.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


