-
iestinstrument
How Temperature Affects Electrode Performance: Optimization Directions from Tortuosity Changes
1. Background
Electrode tortuosity is a key parameter that quantifies the complexity of the internal ion transport pathways within an electrode. It intuitively reflects the ease of ion transport by characterizing the ratio of the actual migration path of lithium ions through the pore network to the theoretical straight-line distance. Research shows a direct correlation between electrode tortuosity and battery rate performance and cycle life: lower tortuosity signifies more efficient ion transport pathways, significantly enhancing the battery’s fast-charging capability. Conversely, excessively high or unstable tortuosity leads to increased polarization, accelerated capacity fade, and seriously impacts cycle stability. In practical applications, temperature, as a critical environmental variable, affects the microstructure and transport properties of electrodes through multiple mechanisms. On one hand, temperature changes alter the viscosity and ionic conductivity of the electrolyte, influencing its wetting behavior within the electrode pores. On the other hand, temperature fluctuations cause changes in the motion state of polymer chains in the binder, subsequently affecting the electrode’s pore structure and mechanical strength.
Therefore, deeply understanding the relationship between temperature and electrode tortuosity not only helps optimize electrode design but also provides theoretical guidance for developing high-performance lithium batteries adapted to extreme environments. This holds significant scientific and engineering value for advancing new energy technologies.
2. Test Conditions & Methods
2.1 Test Equipment
Electrode Tortuosity Testing: The multi-channel ionic conductivity test system (EIC2400M-T) developed by IEST was used, as shown in Figure 1. This device includes four test channels capable of independent temperature control for each channel (range: 10-80°C), provides a high-purity argon atmosphere, and enables electrochemical impedance spectroscopy (EIS) testing of multi-channel symmetric cells. The pressure range is 10–50 kg, and the frequency range is 100 kHz–0.01 Hz.
Figure 1. Multi-channel ionic conductivity test system(EIC2400)
2.2 Test Sample
Graphite negative electrode sheets.
2.3 Test Procedure
For tortuosity testing, samples were assembled in the fixture in the sequence electrode-separator-electrode → chamber closed → quantitative electrolyte injection into each channel → after the wetting period the system automatically collected EIS data → software fitting and calculation were used to derive electrode tortuosity.
2.4 Calculation Method
MacMullin Number Calculation Method:
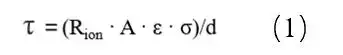
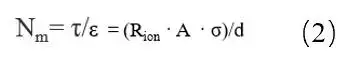
Where: τ is the tortuosity; R_ion is the ionic resistance; A is the electrode area; ε is the electrode porosity; σ is the electrolyte conductivity; d is the electrode thickness. As testing electrode porosity is complex, the ratio of tortuosity to porosity, known as the MacMullin number (N_m = τ / ε), is typically used to characterize electrode tortuosity, as shown in formula (2).
The impedance of the symmetric cell was tested using an electrochemical workstation; the obtained EIS is shown in Figure 3. Extend the low-frequency segment of the Nyquist plot until it intersects the X-axis. Three times the difference between this intersection point and the intersection point of the high-frequency segment with the X-axis gives the ionic resistance R_ion of the electrode coating. Substitute the fitted ionic resistance R_ion into formula (2) to calculate the MacMullin number of the electrode, thereby analyzing its tortuosity.
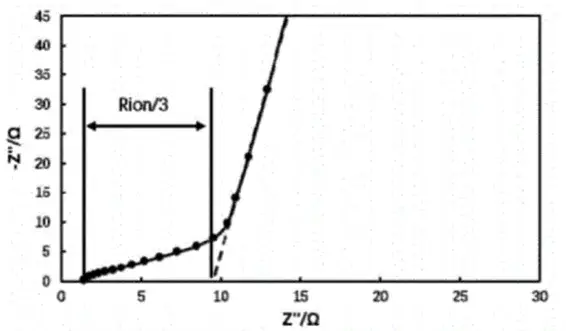
Figure 2. Electrochemical Impedance Spectrum of a Symmetric Cell
3. Results Analysis
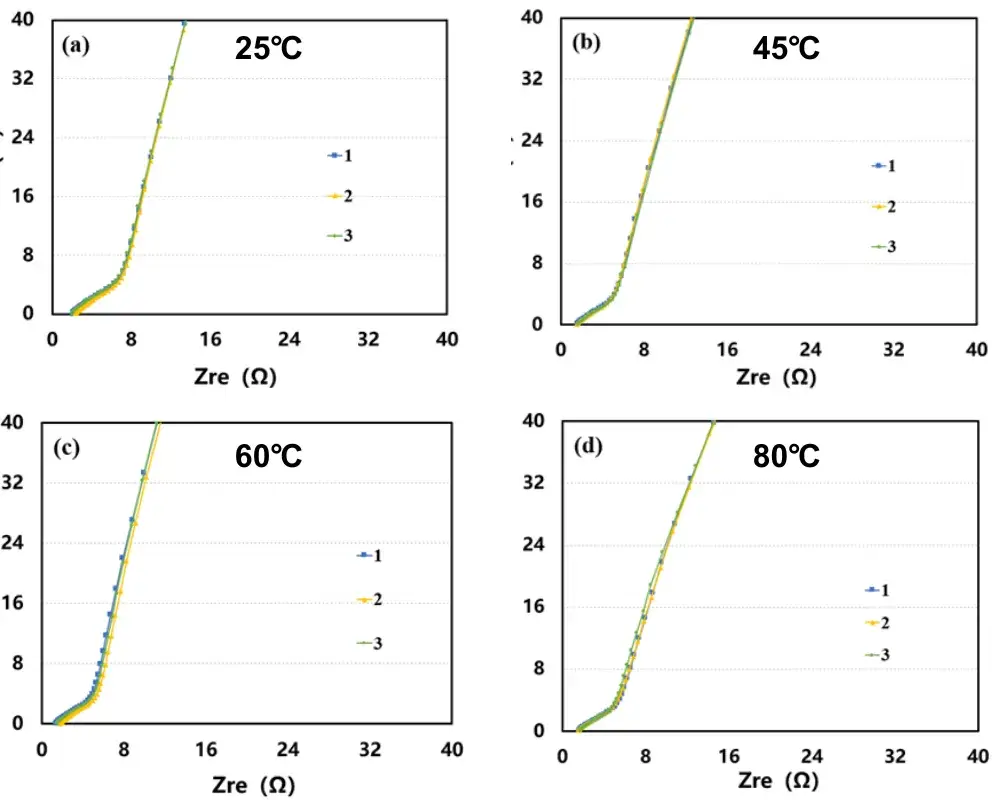
Figure 3. Electrochemical impedance spectra of symmetric cells at different temperatures: (a) 25°C; (b) 45°C; (c) 60°C; (d) 80°C
Table 1. Ionic Resistance and MacMullin Number of the Electrode at Different Temperatures
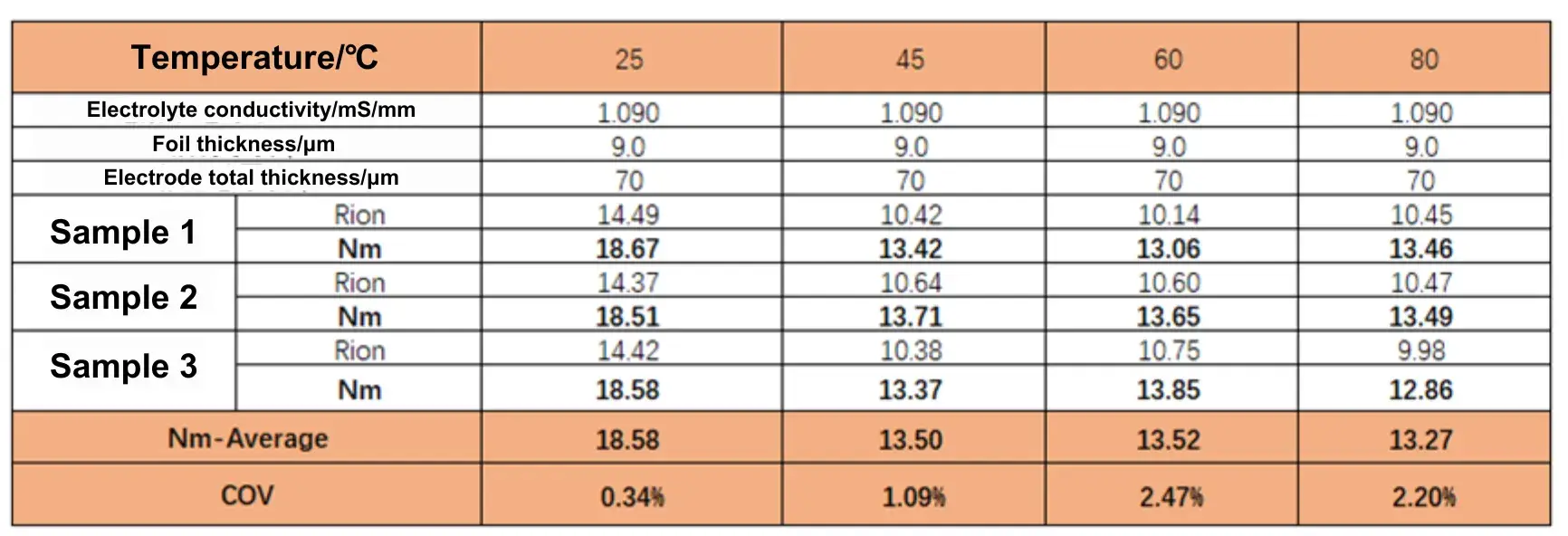
Figure 3 shows EIS spectra measured for the graphite anode symmetric cells at different temperatures: (a) 25°C; (b) 45°C; (c) 60°C; (d) 80°C. Fitting the EIS data produced ionic resistance values for each electrode coating, which were used to compute the MacMullin number; the results are listed in Table 1.
The trend in the data shows that electrode ionic resistance decreases as temperature increases. Electrolyte viscosity is a primary factor influencing ion conductivity: per the Stokes–Einstein relation, ion migration rate is inversely proportional to electrolyte viscosity. Thus, when temperature rises the electrolyte viscosity falls, lithium-ion mobility increases, and electrode ionic resistance decreases.
From the plotted trend (Fig. 4), when temperature increases from 25°C to 45°C the electrode MacMullin number drops from 18.58 to 13.50 — a decrease of 27.34%. However, further increases to 60°C and 80°C show almost no additional reduction relative to 45°C (changes of ≈-0.14% and +1.7%, respectively), indicating minimal improvement in ionic transport at these higher temperatures.
Besides changes in electrolyte viscosity, temperature also affects electrode microstructure. Although lower viscosity at high temperature should reduce ionic resistance, elevated temperature can simultaneously induce structural degradation in the electrode: (1) binder softening (e.g., PVDF softens above ~60°C) can cause pore collapse and pore-size reduction, lowering porosity and lengthening ion pathways; (2) differential thermal expansion between the coating and the current collector can cause interfacial delamination, creating dead zones for ion transport. These degradation mechanisms increase ionic resistance and can offset the transport gains from reduced viscosity, which explains the lack of further resistance decrease in the 60–80°C range. This competing mechanism indicates that electrolyte optimization alone cannot overcome high-temperature performance limits — electrode thermal stability must be improved in parallel.
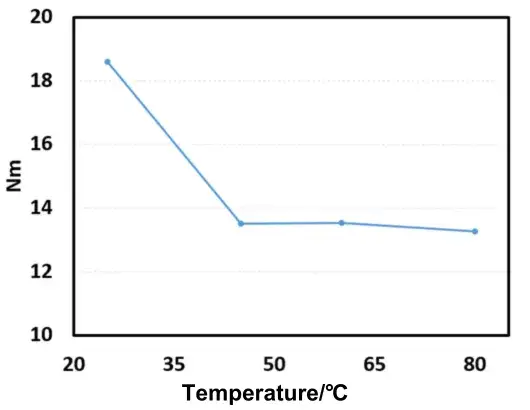
Figure 4. Line Chart of MacMullin Number Changes at Different Temperatures
4. Conclusion
This study experimentally tested the changes in ionic resistance of a graphite negative electrode at different temperatures and found that temperature significantly affects electrode ionic resistance. While the ionic resistance decreases with rising temperature, excessive heat causes significant structural changes in the electrode, preventing further substantial decrease in ionic resistance despite lower electrolyte viscosity. This phenomenon suggests that testing tortuosity changes at different temperatures can provide a rapid method for assessing the high-temperature stability of electrodes.
This finding offers insights for electrode material R&D: On one hand, studying the temperature-impedance relationship can help determine the optimal temperature window for electrode wetting processes. On the other hand, for high-temperature applications, focus should be on developing new material systems with the following characteristics:
- Electrolyte formulations with superior high-temperature stability
- Electrode processes with enhanced resistance to thermal deformation;
- Binder systems resistant to high temperatures. These research directions will contribute to improving lithium battery performance across a wide temperature range.
By testing ionic resistance changes at different temperatures, researchers can establish an effective method for rapidly evaluating the high-temperature stability of electrodes.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



