Description
1. Introduction
1.1 The Significance of Coin Cells
As the new energy industry continues to evolve, the development and testing of new materials are essential. In addition to evaluating conventional physical and chemical properties (such as particle size, BET surface area, XRD, SEM, etc.), it is even more important to conduct preliminary electrochemical performance testing and evaluation of materials and products fabricated with new processes. As a critical step in new-energy R&D, coin cell assembly quality directly determines the accuracy of material performance assessments.
1.2 Type of Coin Cell: CR/BR
Common models are: CR2032/2430/2025/2016, etc. The first 2 digits are diameter (in mm) and the last 2 digits are thickness (in 0.1 mm).
2. The Structure of Coin Cell

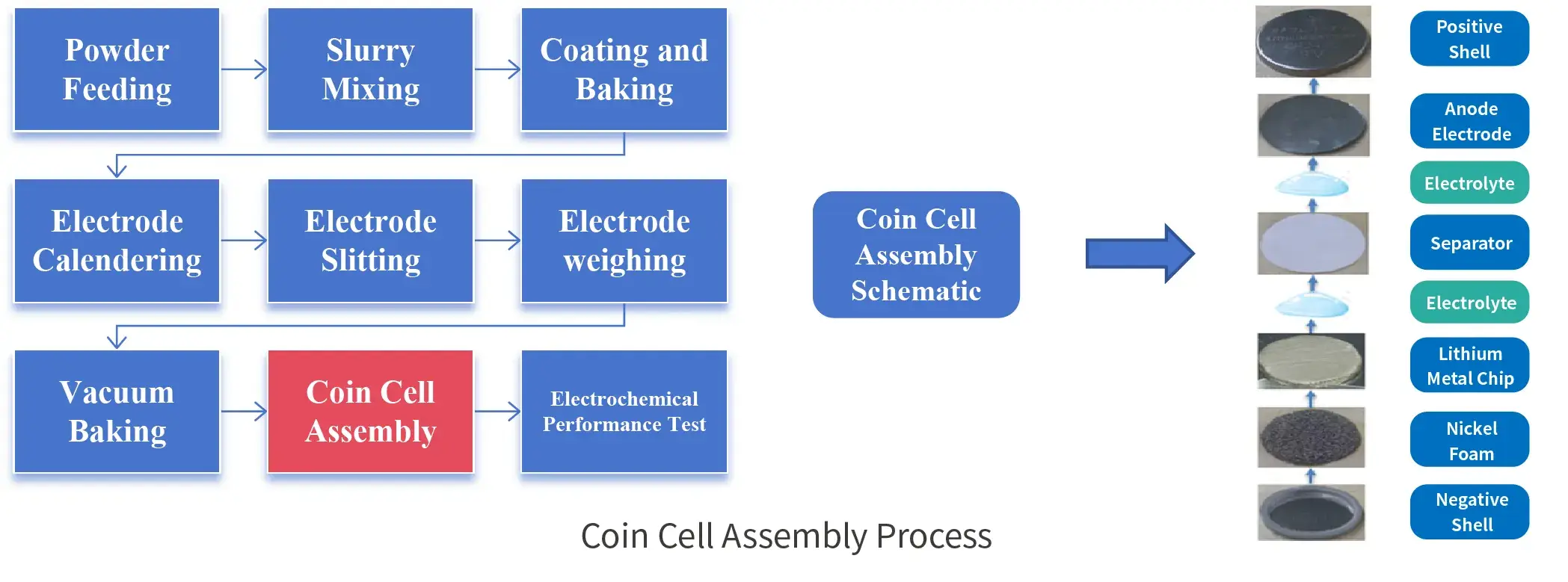
3. Coin Cell Assembly Process
Although the structure of coin cell is relatively simple compared to full cells, the preparation process is the same, which requires slurry coating at the powder end, rolling, punching and weighing of the electrode, coin cell assembly, and finally electrical performance testing.
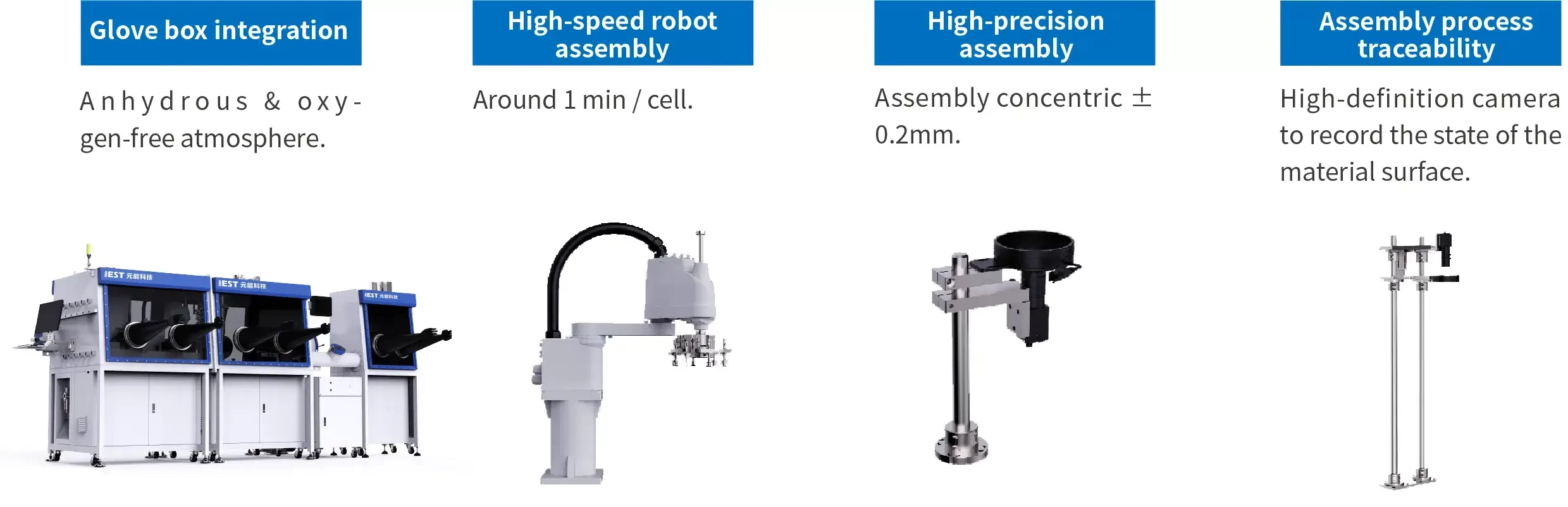
4. Product Introduction: Next-Gen Coin Cell Assembly Solutions For Lab & Industrial-scale
5. Features:
5.1 High-precision Robotic Arms for Precise Grasping
Precise gripping of curled electrodes achieves assembly concentricity within ±0.2mm.
5.2 Electrolyte Automated Switching/Washing
Specialized fixtures for different material types, along with automated electrolyte switching and washing functions, effectively prevent cross-contamination.
5.3 Specialized Material Fixtures
Specialized fixtures for different material types, completely eliminating cross-contamination
5.4 Save Time With High Throughput Assembly
Equipped with a 200-unit material rack, CAAS supports high throughput rapid auto-assembly while minimizing repetitive mechanical operations.
5.5 Automatic Sealing Module
The sealing module ensures precise control over pressure and thickness, significantly improving sealing integrity.
5.6 Full-process intelligent Monitoring
A dual CCD vision system provides real-time monitoring of material status throughout the process, making battery data traceable and transparent.
5.7 Reduce the Skill Threshold for Operators
The Automatic coin cell assembly machine significantly lowers the skill threshold for operators, allowing staff to master coin cell assembly without intensive training.
6. System Features:


Applications
1. Case 1: Application on Curled Electrodes
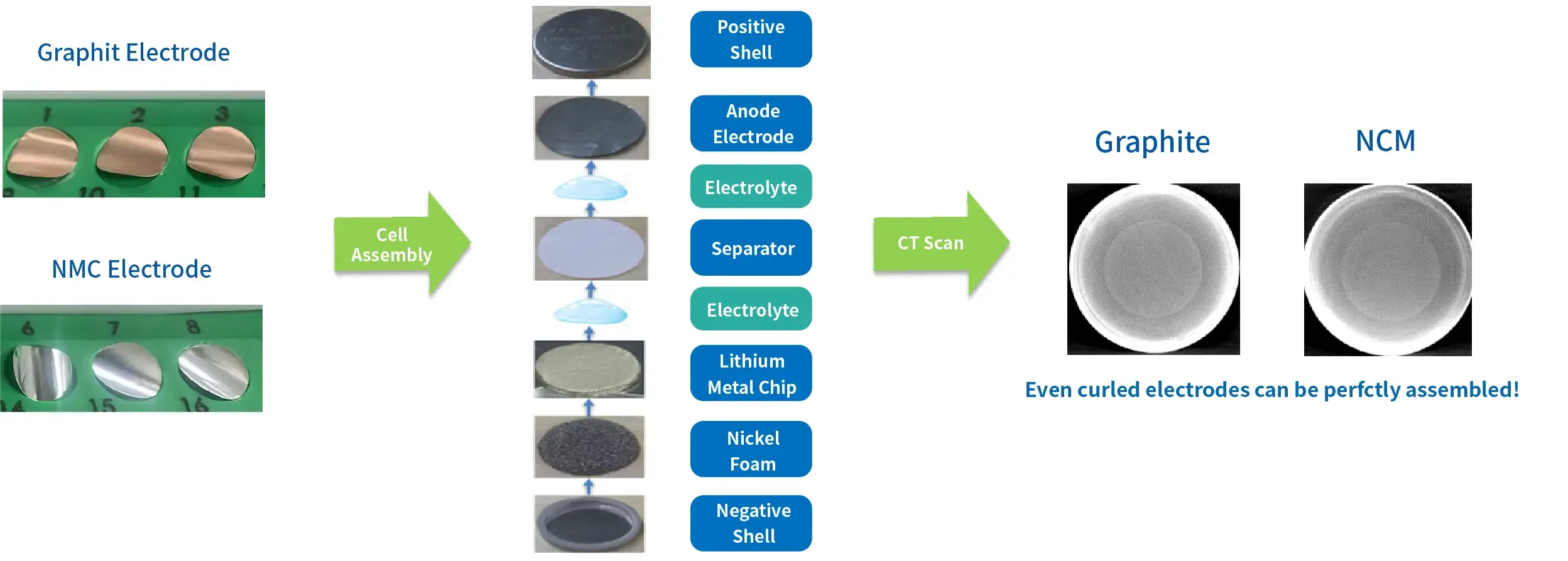
- Our special suction cup can ensure that the curled electrode is sucked evenly and flatly.
- Our visual positioning system can avoid the placement position deviation caused by the curling of the electrodes.
- The positive electrode shell is pressed down horizontally to flatten the curled electrode that contacts the electrolyte.
2. Case 2: Application on the NCM Half Cells
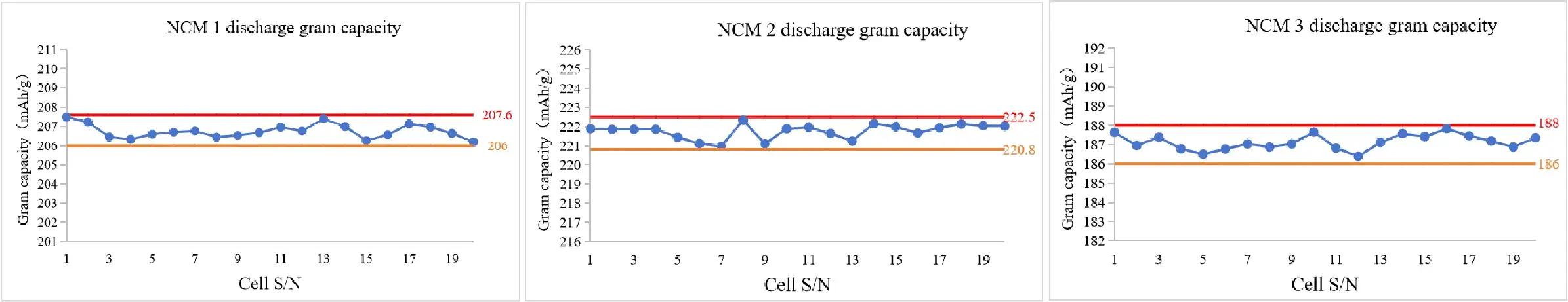
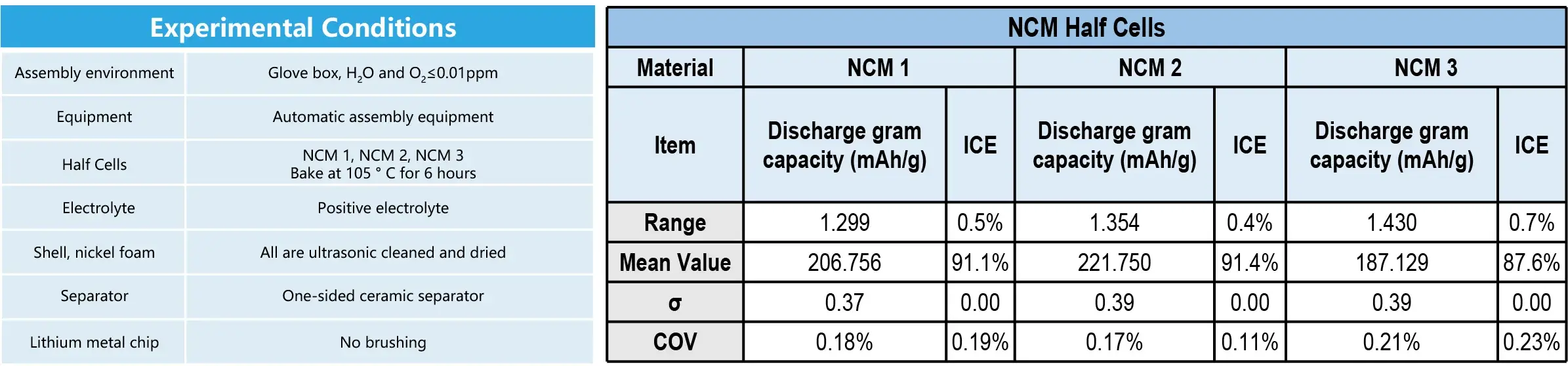
- Conclusion: The range of discharge gram capacity can be controlled within 1.5 mAh/g.
3. Case 3: Application on the LFP Half Cells
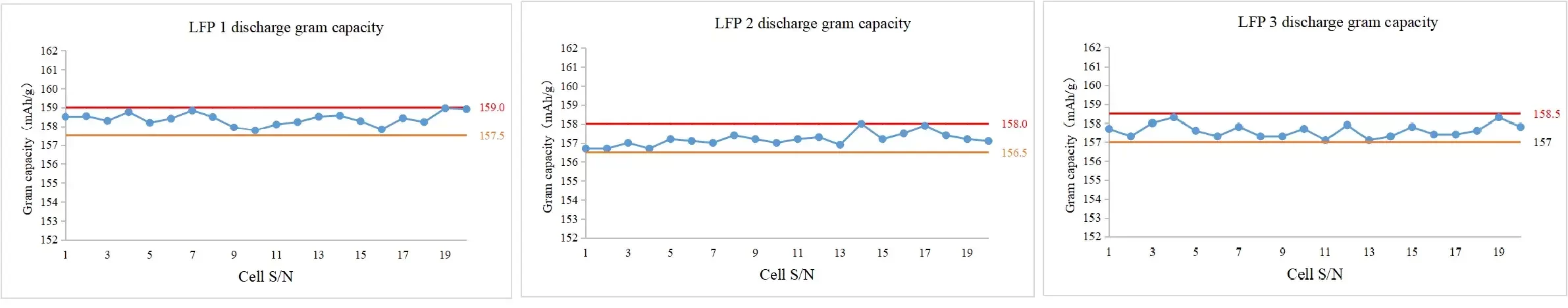
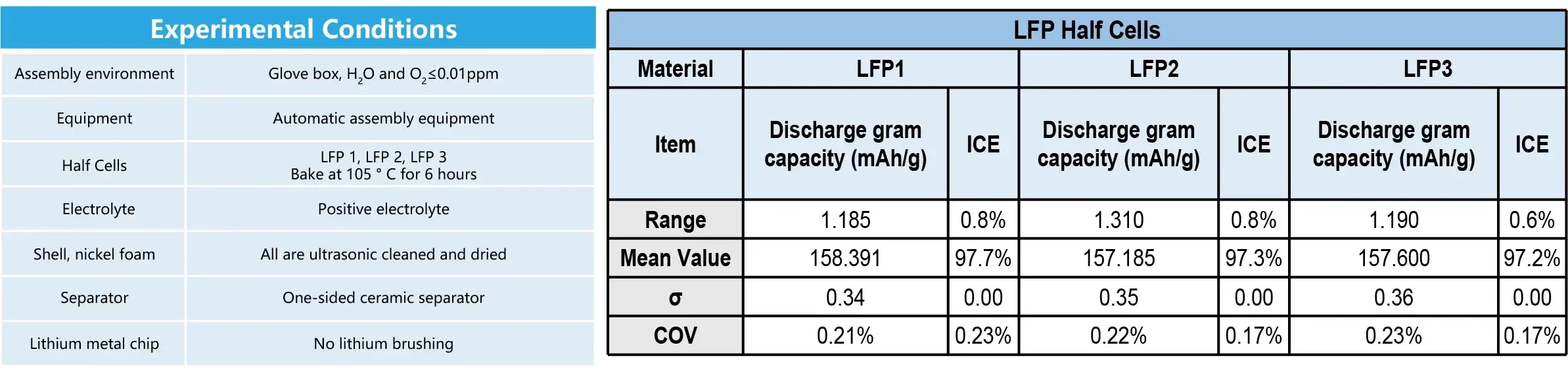
- Conclusion: The range of discharge gram capacity can be controlled within 1.5 mAh/g.
4. Case 4: Application on the Graphite Half Cells
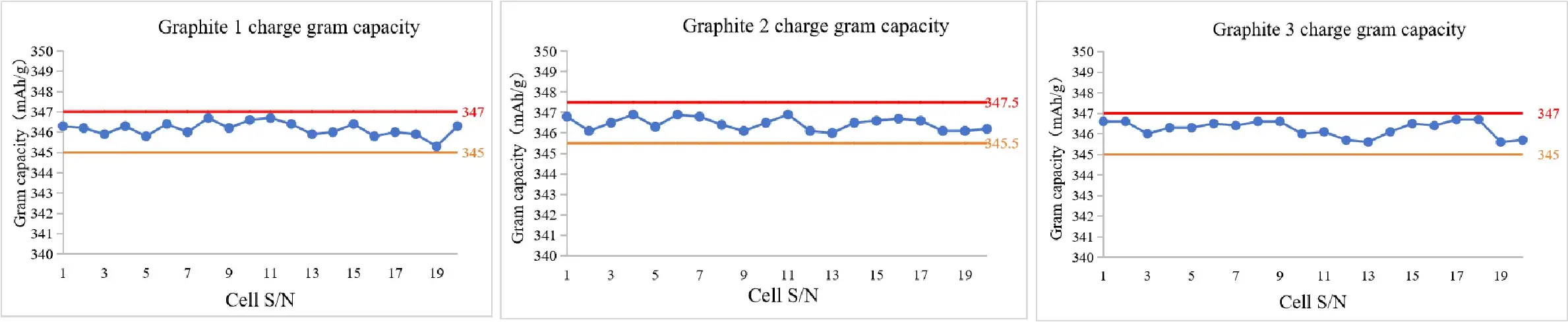
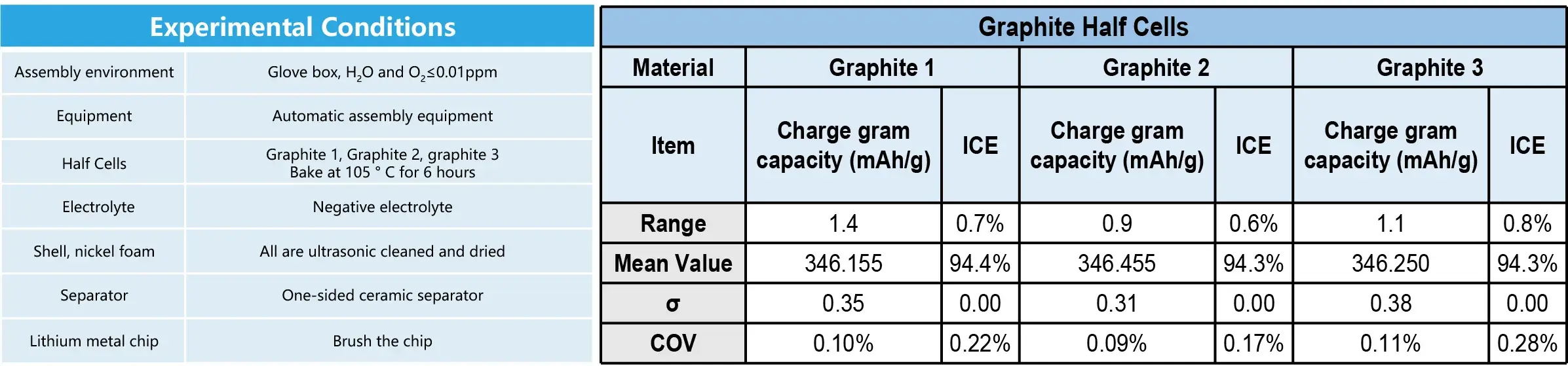
- Conclusion: The range of charge gram capacity can be controlled within 2 mAh/g.
Video













