-
iestinstrument
Analysis of Conductive Carbon Content on Multi-Scale Resistivity in Lithium-Ion Batteries
1. Introduction
Lithium-ion batteries power a wide array of applications, from portable electronics to electric vehicles and grid storage. A critical challenge across these fields is enhancing fast-charging capability, which is intrinsically linked to rate performance. This performance is governed by the total resistance opposing lithium-ion transport during charge and discharge cycles. While research focuses on all cell components, the electronic conduction path within the electrode is paramount. Conductive agents, such as conductive carbon, play a pivotal role by establishing efficient electron transport networks among active material particles. However, their small particle size and low density pose challenges for achieving uniform dispersion in the electrode slurry and coating[1-6].
This study systematically investigates the impact of conductive carbon (Super P, SP) content on electrical resistance across four critical manufacturing stages: powder, slurry, electrode sheet, and coin cell. By quantifying resistance changes at each stage, we provide a clear, data-driven framework for optimizing electrode formulations to achieve superior rate capability.
2. Experimental Materials and Methods
2.1 Materials
The study utilized lithium nickel cobalt manganese oxide (NCM) as the active material, polyvinylidene fluoride (PVDF) as the binder, N-Methyl-2-pyrrolidone (NMP) as the solvent, and Super P carbon black as the conductive agent. Coin cells (2032-type) were assembled with lithium metal as the counter electrode.
2.2 Analysis and Test Instrument
Four probe powder powder resistivity & compaction density(PRCD2100), four probe mode, battery slurry resistance Tester(BSR2300), battery electrode resistance tester(BER2500), the above three equipment are from IEST; Battery Tester (CT-4008T-Neware), Electrochemical Workstation (DH7001).
2.3 Experimental Methods
According to the formula ratio shown in Table 1, five groups of cathode paste, electrode sheet and buckle batteries were prepared. Different test equipment were used to test the resistance performance of slurry, electrode sheet and coin cells respectively, and then the influence of conductive carbon content change on the electrical performance of each level was analyzed.
Table 1. Percentage of Mass of the Five Sample Groups
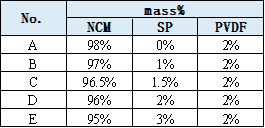
2.4 Sample Preparation
Call the materials according to the proportion of each group of materials in Table 1, mix the high-speed mixing defoam machine for 11 minutes, and some use the semi-automatic comachine to apply them to the aluminum foil. After drying, some of the electrode parts, the electrode half is used for the assembly of buckle battery. The buckle battery is assembled in an argon glove box, with the ternary electrode sheet positive and the lithium sheet negative.
3. Results and Discussion: A Layer-by-Layer Analysis
3.1 Analysis of Powder Layer
Initial measurements on the raw materials established a baseline. As shown in Figure 1, the resistivity of carbon black (SP) was drastically lower than that of NCM. At a compaction density of 1.0 g/cm³, SP exhibited a resistivity of approximately 0.02 Ω·cm. In contrast, NCM reached a resistivity of about 16.7 Ω·cm at 3.5 g/cm³. Therefore, in the powder level, the ternary material resistance is 835 times that of the conductive carbon, and the conductive carbon conductivity is much better than that of the ternary material, which will affect the conductivity of the subsequent slurry and electrode.

Figure 1. (a) powder compaction density change with test pressure intensity

Figure 1. (b) powder resistivity versus compaction density
3.2 Slurry and Electrode Sheet-Level Resistivity
Figure 2 (a) for the test results of five groups of slurry resistivity, as can be seen from the figure, the slurry resistivity is reduced with the increase of conductive carbon content, this is because when the conductive carbon content increases, in the slurry suspension of ternary particles between more conductive carbon particles connection, so the electron transmission between the particles is faster, the resistivity is smaller.Figure 2 (b) shows the test results of the electrode resistivity before and after the five sets of roller pressure. It can be seen from the figure that the electrode resistivity decreases with the increase of the conductive carbon content, which shows that the increase of the conductive carbon content will significantly improve the electronic conductivity between the particles. In addition, due to the closer contact between the particles and the coating layer and the fluid collector after the roller pressure, the resistivity value of the electrode plate is one order of magnitude lower than that before the roller pressure, which also indicates that the roller pressure will significantly improve the electrical conductivity of the positive electrode plate.

Figure 2. (a) resistivity curve of five slurry groups

Figure 2. (b) resistivity curve of five electrodes
3.3 Coin Cell-Level Electrochemical Performance
The AC impedance spectroscopy test and multiplier performance test of the five groups of buckle batteries after charging and discharging one activation were conducted, and the results are shown in Figure 3 (a), 3 (b) and 3 (c). In lithium-ion battery systems, the medium to high frequency range in the impedance spectrum represents electron transfer and charge transfer, and the low frequency range represents ion diffusion[7]. As can be seen from Figure 3 (b), with the increase of the battery transfer carbon content from 0% to 3%, the sum of the electron transfer Rs and the charge transfer resistance R ct also gradually decreases, which shows that the amount of conductive carbon added has a significant positive effect on the improvement of the battery resistance. In addition, if only the electronic resistance at the high frequency is compared, it will be affected by the contact resistance of the buckle battery shell and the electrode sheet, and the change trend of the first two groups is not consistent with the change of the conductive carbon content. According to the different ratio discharge capacity retention rate in Figure 3 (c), as the discharge ratio gradually increases to 2.5 C, when the conductive carbon content is less than 1%, the discharge capacity is almost less than 2%, while when the conductive carbon content is more than 1.5%, the discharge capacity of the battery remains above 80%. Therefore, this demonstrates that an adequate amount of conductive agent is not merely beneficial but essential for realizing high-rate capability.

Figure 3. (a) EIS curves for five sets of button cells

Figure 3. (b) Electronic and ionic resistance profiles of five sets of button cell batteries

Figure 3. (c) Discharge retention curves of five sets of button cell batteries with different multiplication rates
4. Cross-scale synthesis: Why Multi-level Measurement Matters
This study’s four-level approach — powder → slurry → electrode (before/after calender) → coin cell — highlights how a low-resistivity additive at the powder stage becomes influential only if it is well dispersed and preserved through coating and calendering. Improvements seen at the slurry and electrode levels do translate to cell-level benefits, but only when mechanical and electrochemical interfaces are well controlled. Therefore, formulation engineers should combine quantitative resistivity screening at each stage with process controls (mixing energy, dispersion protocol, calender pressure) to achieve reliable rate performance.
5. Conclusion
Adding conductive carbon markedly reduces resistivity across powder, slurry and electrode scales, and these reductions produce measurable improvements in coin-cell rate performance. The data indicate a practical lower bound for conductive carbon in the tested system (≈1.5 wt%) to sustain ≥80% capacity at 2.5 C, while calendering further amplifies the conductivity benefits. By combining multi-scale resistivity screening with disciplined dispersion and calender protocols, teams can tune formulations to optimize both energy density and fast-charge capability.
6. References
[1] Xu Jieru, Li Hong, et al. Conductivity test and analysis methods for research of lithium batteries [J]. Energy Storage Science and Technology, 2018,7 (5): 926-955.
[2] Kondo H, Sawada H, Okuda C, et al. Influence of the active material on the electronic conductivity of the positive electrode in lithium-ion batteries [J].Journal of the Electrochemical Society, 2019, 166(8): A1285-A1290.
[3] Nie Lei, Qin Xing, Zhang Na, et al. Research on Lithium-ion Battery [J]. Power supply technology, 2019,43 (4): 562-563.
[4] Westphal B G, Mainusch N, Meyer C, et al. Influence of high intensive dry mixing and calendering on relative electrode resistivity determined via an advanced two point approach [J].Journal of Energy Storage, 2017, 11:76-85.
[5] Mainusch N, Christ T, Siedenburg T, et al. New Contact Probe and Method to Measure Electrical Resistances in Battery Electrodes [J].Energy Technology, 2016, 4, 1550-1557
[6] Liao Xiaodong, Huang Ju, Wang Ronggui. Effect of cathode conductive carbon content on the performance of lithium-ion batteries [J]. Dongfang Electric Review, 2013,27 (105): 4-7.
[7] Zhuang Quanchao, Xu Shoudong, Qiu Xiangyun, et al. Electrochemical impedance spectrometry analysis of lithium-ion batteries [J]. Chemical Advances, 2010,22 (6): 1044-1057.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


